
PDF Publication Title:
Text from PDF Page: 004
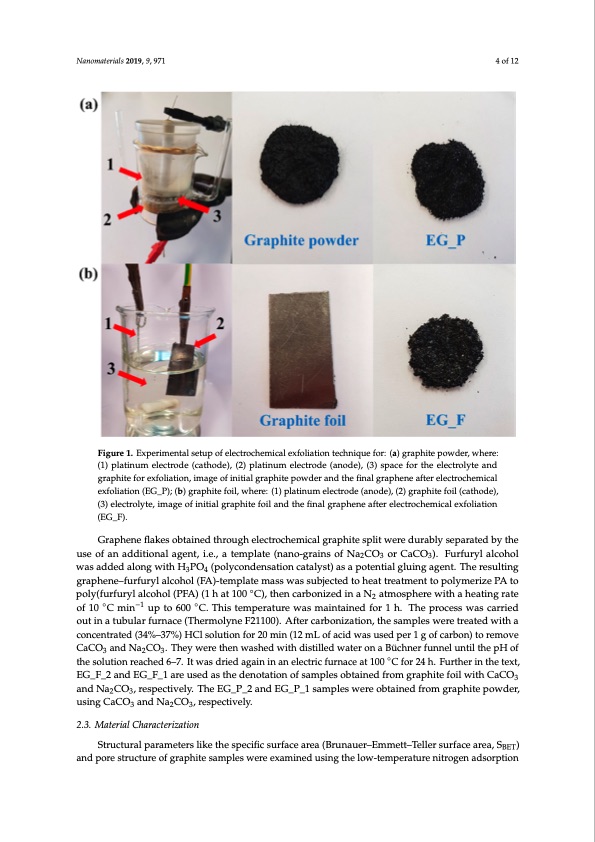
Nanomaterials 2019, 9, 971 4 of 12 Nanomaterials 2019, 9, x FOR PEER REVIEW 4 of 12 Figure 1. Experimental setup of electrochemical exfoliation technique for: (a) graphite powder, where: Figure 1. Experimental setup of electrochemical exfoliation technique for: (a) graphite powder, where: (1) platinum electrode (cathode), (2) platinum electrode (anode), (3) space for the electrolyte and (1) platinum electrode (cathode), (2) platinum electrode (anode), (3) space for the electrolyte and graphite for exfoliation, image of initial graphite powder and the final graphene after electrochemical graphite for exfoliation, image of initial graphite powder and the final graphene after electrochemical exfoliation (EG_P); (b) graphite foil, where: (1) platinum electrode (anode), (2) graphite foil (cathode), exfoliation (EG_P); (b) graphite foil, where: (1) platinum electrode (anode), (2) graphite foil (cathode), (3) electrolyte, image of initial graphite foil and the final graphene after electrochemical exfoliation (3) electrolyte, image of initial graphite foil and the final graphene after electrochemical exfoliation ((EG__FF)).. ® AGdrairpehcetncuerflraeknets(DoCbt)avinoeltdagther(oRu&gSh eHleMctPro20ch20empoicwalergrsauphpiltye)swplaist awpeprelieddubraebtwlyeseenptahreatpeldatbinyutmhe aunsdegorfapanhitaededlietciotrnoadleasg, aendt, tih.e.,elaecttermolpylsaitsep(rnoacnedo-ugrrealiansteodf 2Nha2foCrOg3raoprhCitaeCpOow3)d. eFruarnfdur3y0lmalicnofhoorl gwrapshaidtedfeodilaaltornogowmithemHp3ePrOat4u(rpeo. Dlyucorindgetnhsiastpiornocceastsa,lsyusbt)satasnacepsoetexnpteicatlegdlutoinwgoargkeanst.aTsheeparerastuolrtionfg ngerwaplyhenxefo–lfiuartfeudryglraplchoehnoel (fFlaAk)e-ste(mdepnlaotedmlaastserwas tseumbjpelcatteeds)towheeraetatdredaetdmeintotnoepoflytmwoerfizoermPAs: tao ◦ ◦ −1 ◦ of 10GraCpmheine flaukpestob6t0a0ineCd. tThhroisutgehmeplecrtartoucrheemwaicsalmgarianpthainteesdpfliotrw1ehre. dTuhreabplyroscepsasrwataesd cbayrrtiheed uosuetoinfantuabdudliatirofnuarlnagcen(tT,hi.er.,maotleymneplFa2t1e1(0n0a)n.oA-gftrearincsarobfoNniaz2aCtiOo3no,rthCeasCamO3p).leFsuwrfeurreytlraelactoehdowlwithasa acdodnecdenatrlaotnegd(w34it%h–H373%PO)H4C(plosloylcuotinodnefnosra2ti0omnicna(t1a2lymstL)oasfaacidpowteanstuiasledglpueirng1gagoefncat.rbTohne)troesruemltionvge gCrapChOenaen–dfuNrfua rCylOal.coThoely(wFAer)e-ttehmenplwataesmheadsswwithasdsiustbiljleecdtewdattoerhoeantatrBeüactmhneenrtftuonpnoelyumnteirlitzheePpAHtof 323 ◦ −1 1E0G°C_Fm_2inandupEGto_F60_01 °aCre. Tuhseisdtaesmtpherdateunroetawtiaosnmoafisnatmainpeledsfobrt1aihn.eTdhferopmrogcerassphwitaesfcoairlrwieidthoCutaCinOa taunbdulNara fCuOrna,cresp(Tehcteirvmeloyl.yTnheeFE2G1_10P0_)2. aAnfdteErGc_aPr_b1onsaizmatpiolens,wtheree osabmtapinlesd fwroemre gtraepahteidte pwoiwthdear, 23 cuosnicnegnCtraCteOd (3a4n%d–N37a%C)OHC, rlessopluectitoivnefloy.r 20 min (12 mL of acid was used per 1 g of carbon) to remove 323 CaCO3 and Na2CO3. They were then washed with distilled water on a Büchner funnel until the pH of npaonloy-(pfuorwfudreyrloaflcCoahCoOl (3P(F5A–4)0(1nmh adti1a0m0eteCr),othreansacaturbraotneidzesdoluintiaonNofaNtma2oCsOph3.ere with a heating rate 2 ptohleys(ofulurftuiornylreaalcohheodl6(–P7F.AIt) w(1ahs adtri1e0d0a°gCa)i,nthinenancaerlbeoctnrizcefdurinaaceNa2ta1t0m0osCphfoere24whit.hFaurhtheaetriningtrhaeteteoxft, 2.3. Material Characterization the solution reached 6–7. It was dried again in an electric furnace at 100 °C for 24 h. Further in the text, EG_F_2 and EG_F_1 are used as the denotation of samples obtained from graphite foil with Structural parameters like the specific surface area (Brunauer–Emmett–Teller surface area, SBET) CaCO3 and Na2CO3, respectively. The EG_P_2 and EG_P_1 samples were obtained from graphite and pore structure of graphite samples were examined using the low-temperature nitrogen adsorption powder, using CaCO3 and Na2CO3, respectively. 2.3. Material Characterization 3PDF Image | Electro-Exfoliation of Graphite to Graphene
PDF Search Title:
Electro-Exfoliation of Graphite to GrapheneOriginal File Name Searched:
graphene-aqueous-salt-al.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |