
PDF Publication Title:
Text from PDF Page: 004
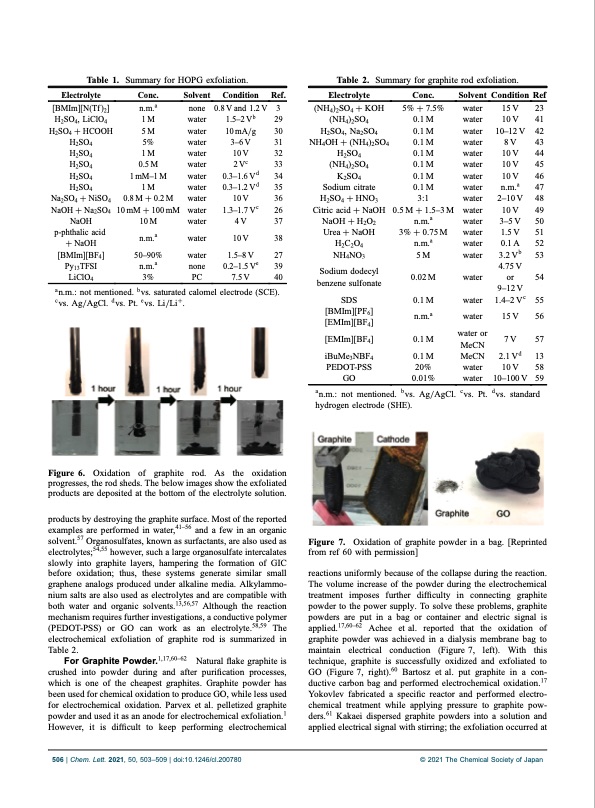
Table 1. Summary for HOPG exfoliation. Electrolyte Conc. Solvent Condition Ref. Table 2. Summary for graphite rod exfoliation. Electrolyte (NH4)2SO4 + KOH (NH4)2SO4 H2SO4, Na2SO4 NH4OH + (NH4)2SO4 H2SO4 (NH4)2SO4 K2SO4 Sodium citrate H2SO4 + HNO3 Citric acid + NaOH NaOH + H2O2 Urea + NaOH H2C2O4 NH4NO3 Sodium dodecyl benzene sulfonate SDS [BMIm][PF6] [EMIm][BF4] [EMIm][BF4] iBuMe3NBF4 PEDOT-PSS GO Conc. 5% + 7.5% 0.1 M 0.1 M 0.1 M 0.1 M 0.1 M 0.1 M 0.1 M 3:1 0.5 M + 1.53 M n.m.a 3% + 0.75 M n.m.a 5 M 0.02 M 0.1 M n.m.a 0.1 M 0.1 M 20% 0.01% Solvent Condition Ref n.m.a 1 M 5 M 5% 1 M 0.5 M 1 mM1 M 1 M 0.8 M + 0.2 M 10 mM + 100 mM 10 M n.m.a an.m.: not mentioned. bvs. saturated calomel electrode (SCE). cvs. Ag/AgCl. dvs. Pt. evs. Li/Li+. Figure 6. Oxidation of graphite rod. As the oxidation progresses, the rod sheds. The below images show the exfoliated products are deposited at the bottom of the electrolyte solution. products by destroying the graphite surface. Most of the reported examples are performed in water,4156 and a few in an organic solvent.57 Organosulfates, known as surfactants, are also used as electrolytes;54,55 however, such a large organosulfate intercalates slowly into graphite layers, hampering the formation of GIC before oxidation; thus, these systems generate similar small graphene analogs produced under alkaline media. Alkylammo- nium salts are also used as electrolytes and are compatible with both water and organic solvents.13,56,57 Although the reaction mechanism requires further investigations, a conductive polymer (PEDOT-PSS) or GO can work as an electrolyte.58,59 The electrochemical exfoliation of graphite rod is summarized in Table 2. For Graphite Powder.1,17,6062 Natural flake graphite is crushed into powder during and after purification processes, which is one of the cheapest graphites. Graphite powder has been used for chemical oxidation to produce GO, while less used for electrochemical oxidation. Parvex et al. pelletized graphite powder and used it as an anode for electrochemical exfoliation.1 However, it is difficult to keep performing electrochemical [BMIm][N(Tf )2] H2SO4, LiClO4 H2SO4 + HCOOH H2SO4 H2SO4 H2SO4 H2SO4 H2SO4 Na2SO4 + NiSO4 NaOH + Na2SO4 NaOH p-phthalic acid + NaOH [BMIm][BF4] Py13TFSI none water water water water water water water water water water water 0.8Vand1.2V 1.52 Vb 10 mA/g 36 V 10V 2Vc 0.31.6 Vd 0.31.2 Vd 10V 1.31.7 Vc 4V 10V 1.58 V 3 29 30 31 32 33 34 35 36 26 37 38 27 39 40 water water water water water water water water water water water water water water water water water water or MeCN MeCN water water 15V 23 10V 41 1012 V 42 8V 43 10V 44 10V 45 10V 46 n.m.a 47 210 V 48 10V 49 35 V 50 1.5 V 51 0.1 A 52 3.2Vb 53 4.75 V or 54 912 V 1.42 V c 55 15V 56 7 V 57 2.1 Vd 13 10 V 58 10100 V 59 5090% n.m.a water none 0.21.5 Ve LiClO4 3% PC 7.5V an.m.: not mentioned. bvs. Ag/AgCl. cvs. Pt. dvs. standard hydrogen electrode (SHE). Figure 7. Oxidation of graphite powder in a bag. [Reprinted from ref 60 with permission] reactions uniformly because of the collapse during the reaction. The volume increase of the powder during the electrochemical treatment imposes further difficulty in connecting graphite powder to the power supply. To solve these problems, graphite powders are put in a bag or container and electric signal is applied.17,6062 Achee et al. reported that the oxidation of graphite powder was achieved in a dialysis membrane bag to maintain electrical conduction (Figure 7, left). With this technique, graphite is successfully oxidized and exfoliated to GO (Figure 7, right).60 Bartosz et al. put graphite in a con- ductive carbon bag and performed electrochemical oxidation.17 Yokovlev fabricated a specific reactor and performed electro- chemical treatment while applying pressure to graphite pow- ders.61 Kakaei dispersed graphite powders into a solution and applied electrical signal with stirring; the exfoliation occurred at 506 | Chem. Lett. 2021, 50, 503–509 | doi:10.1246/cl.200780 © 2021 The Chemical Society of JapanPDF Image | Electrochemical Production of Graphene Analogs
PDF Search Title:
Electrochemical Production of Graphene AnalogsOriginal File Name Searched:
cl-200780.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |