PDF Publication Title:
Text from PDF Page: 005
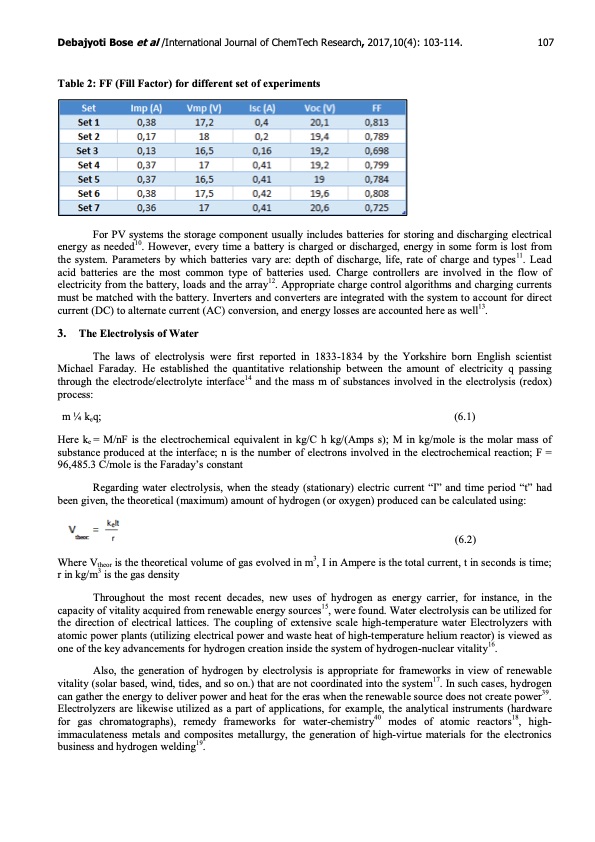
Debajyoti Bose et al /International Journal of ChemTech Research, 2017,10(4): 103-114. 107 Table 2: FF (Fill Factor) for different set of experiments For PV systems the storage component usually includes batteries for storing and discharging electrical energy as needed10. However, every time a battery is charged or discharged, energy in some form is lost from the system. Parameters by which batteries vary are: depth of discharge, life, rate of charge and types11. Lead acid batteries are the most common type of batteries used. Charge controllers are involved in the flow of electricity from the battery, loads and the array12. Appropriate charge control algorithms and charging currents must be matched with the battery. Inverters and converters are integrated with the system to account for direct current (DC) to alternate current (AC) conversion, and energy losses are accounted here as well13. 3. The Electrolysis of Water The laws of electrolysis were first reported in 1833-1834 by the Yorkshire born English scientist Michael Faraday. He established the quantitative relationship between the amount of electricity q passing through the electrode/electrolyte interface14 and the mass m of substances involved in the electrolysis (redox) process: m 1⁄4 keq; (6.1) Here ke = M/nF is the electrochemical equivalent in kg/C h kg/(Amps s); M in kg/mole is the molar mass of substance produced at the interface; n is the number of electrons involved in the electrochemical reaction; F = 96,485.3 C/mole is the Faraday’s constant Regarding water electrolysis, when the steady (stationary) electric current “I” and time period “t” had been given, the theoretical (maximum) amount of hydrogen (or oxygen) produced can be calculated using: (6.2) Where Vtheor is the theoretical volume of gas evolved in m3, I in Ampere is the total current, t in seconds is time; r in kg/m3 is the gas density Throughout the most recent decades, new uses of hydrogen as energy carrier, for instance, in the capacity of vitality acquired from renewable energy sources15, were found. Water electrolysis can be utilized for the direction of electrical lattices. The coupling of extensive scale high-temperature water Electrolyzers with atomic power plants (utilizing electrical power and waste heat of high-temperature helium reactor) is viewed as one of the key advancements for hydrogen creation inside the system of hydrogen-nuclear vitality16. Also, the generation of hydrogen by electrolysis is appropriate for frameworks in view of renewable vitality (solar based, wind, tides, and so on.) that are not coordinated into the system17. In such cases, hydrogen can gather the energy to deliver power and heat for the eras when the renewable source does not create power39. Electrolyzers are likewise utilized as a part of applications, for example, the analytical instruments (hardware for gas chromatographs), remedy frameworks for water-chemistry40 modes of atomic reactors18, high- immaculateness metals and composites metallurgy, the generation of high-virtue materials for the electronics business and hydrogen welding19.PDF Image | Renewable Electrolysis using Graphene electrodes
PDF Search Title:
Renewable Electrolysis using Graphene electrodesOriginal File Name Searched:
V10N4CT.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com (Standard Web Page)