PDF Publication Title:
Text from PDF Page: 006
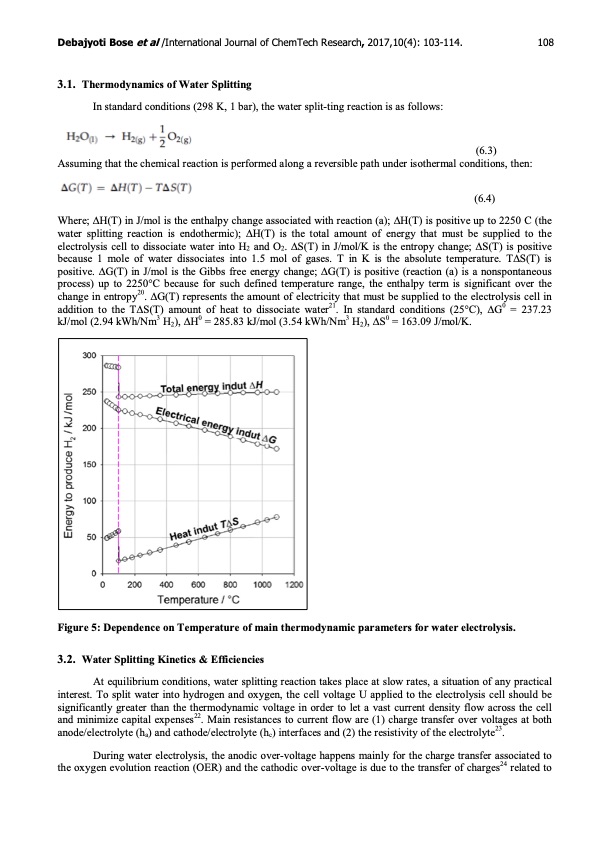
Debajyoti Bose et al /International Journal of ChemTech Research, 2017,10(4): 103-114. 3.1. Thermodynamics of Water Splitting In standard conditions (298 K, 1 bar), the water split-ting reaction is as follows: (6.3) Assuming that the chemical reaction is performed along a reversible path under isothermal conditions, then: (6.4) 108 Where; ∆H(T) in J/mol is the enthalpy change associated with reaction (a); ∆H(T) is positive up to 2250 C (the water splitting reaction is endothermic); ∆H(T) is the total amount of energy that must be supplied to the electrolysis cell to dissociate water into H2 and O2. ∆S(T) in J/mol/K is the entropy change; ∆S(T) is positive because 1 mole of water dissociates into 1.5 mol of gases. T in K is the absolute temperature. T∆S(T) is positive. ∆G(T) in J/mol is the Gibbs free energy change; ∆G(T) is positive (reaction (a) is a nonspontaneous process) up to 2250°C because for such defined temperature range, the enthalpy term is significant over the change in entropy20. ∆G(T) represents the amount of electricity that must be supplied to the electrolysis cell in addition to the T∆S(T) amount of heat to dissociate water21. In standard conditions (25°C), ∆G0 = 237.23 kJ/mol (2.94 kWh/Nm3 H2), ∆H0 = 285.83 kJ/mol (3.54 kWh/Nm3 H2), ∆S0 = 163.09 J/mol/K. Figure 5: Dependence on Temperature of main thermodynamic parameters for water electrolysis. 3.2. Water Splitting Kinetics & Efficiencies At equilibrium conditions, water splitting reaction takes place at slow rates, a situation of any practical interest. To split water into hydrogen and oxygen, the cell voltage U applied to the electrolysis cell should be significantly greater than the thermodynamic voltage in order to let a vast current density flow across the cell and minimize capital expenses22. Main resistances to current flow are (1) charge transfer over voltages at both anode/electrolyte (ha) and cathode/electrolyte (hc) interfaces and (2) the resistivity of the electrolyte23. During water electrolysis, the anodic over-voltage happens mainly for the charge transfer associated to the oxygen evolution reaction (OER) and the cathodic over-voltage is due to the transfer of charges24 related toPDF Image | Renewable Electrolysis using Graphene electrodes
PDF Search Title:
Renewable Electrolysis using Graphene electrodesOriginal File Name Searched:
V10N4CT.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com (Standard Web Page)