PDF Publication Title:
Text from PDF Page: 004
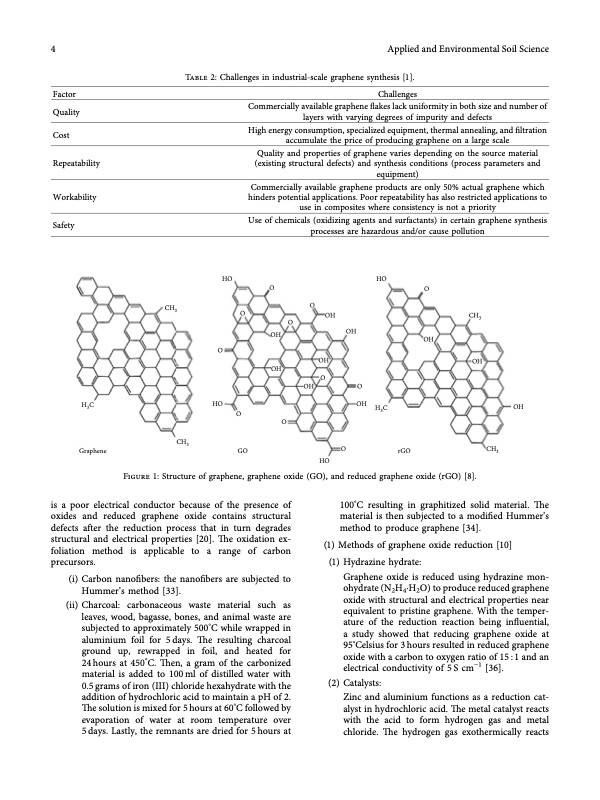
4 Applied and Environmental Soil Science Factor Quality Cost Repeatability Workability Safety Table 2: Challenges in industrial-scale graphene synthesis [1]. Challenges Commercially available graphene fakes lack uniformity in both size and number of layers with varying degrees of impurity and defects High energy consumption, specialized equipment, thermal annealing, and fltration accumulate the price of producing graphene on a large scale Quality and properties of graphene varies depending on the source material (existing structural defects) and synthesis conditions (process parameters and equipment) Commercially available graphene products are only 50% actual graphene which hinders potential applications. Poor repeatability has also restricted applications to use in composites where consistency is not a priority Use of chemicals (oxidizing agents and surfactants) in certain graphene synthesis processes are hazardous and/or cause pollution HO O HO OO CH3 O O OH O O OH OH OH O OH O CH3 OH OH OH H3C HO CH3 Graphene GO OH H3C OH O Figure 1: Structure of graphene, graphene oxide (GO), and reduced graphene oxide (rGO) [8]. is a poor electrical conductor because of the presence of oxides and reduced graphene oxide contains structural defects after the reduction process that in turn degrades structural and electrical properties [20]. Te oxidation ex- foliation method is applicable to a range of carbon precursors. (i) Carbon nanofbers: the nanofbers are subjected to Hummer’s method [33]. (ii) Charcoal: carbonaceous waste material such as leaves, wood, bagasse, bones, and animal waste are subjected to approximately 500°C while wrapped in aluminium foil for 5days. Te resulting charcoal ground up, rewrapped in foil, and heated for 24 hours at 450°C. Ten, a gram of the carbonized material is added to 100 ml of distilled water with 0.5 grams of iron (III) chloride hexahydrate with the addition of hydrochloric acid to maintain a pH of 2. Te solution is mixed for 5 hours at 60°C followed by evaporation of water at room temperature over 5 days. Lastly, the remnants are dried for 5 hours at 100°C resulting in graphitized solid material. Te material is then subjected to a modifed Hummer’s method to produce graphene [34]. (1) Methods of graphene oxide reduction [10] (1) Hydrazine hydrate: Graphene oxide is reduced using hydrazine mon- ohydrate (N2H4·H2O) to produce reduced graphene oxide with structural and electrical properties near equivalent to pristine graphene. With the temper- ature of the reduction reaction being infuential, a study showed that reducing graphene oxide at 95°Celsius for 3 hours resulted in reduced graphene oxide with a carbon to oxygen ratio of 15 : 1 and an electrical conductivity of 5 S cm−1 [36]. (2) Catalysts: Zinc and aluminium functions as a reduction cat- alyst in hydrochloric acid. Te metal catalyst reacts with the acid to form hydrogen gas and metal chloride. Te hydrogen gas exothermically reacts HO O rGO CH3PDF Image | State-of-the-Art Graphene Synthesis Methods
PDF Search Title:
State-of-the-Art Graphene Synthesis MethodsOriginal File Name Searched:
8475504.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com (Standard Web Page)