PDF Publication Title:
Text from PDF Page: 005
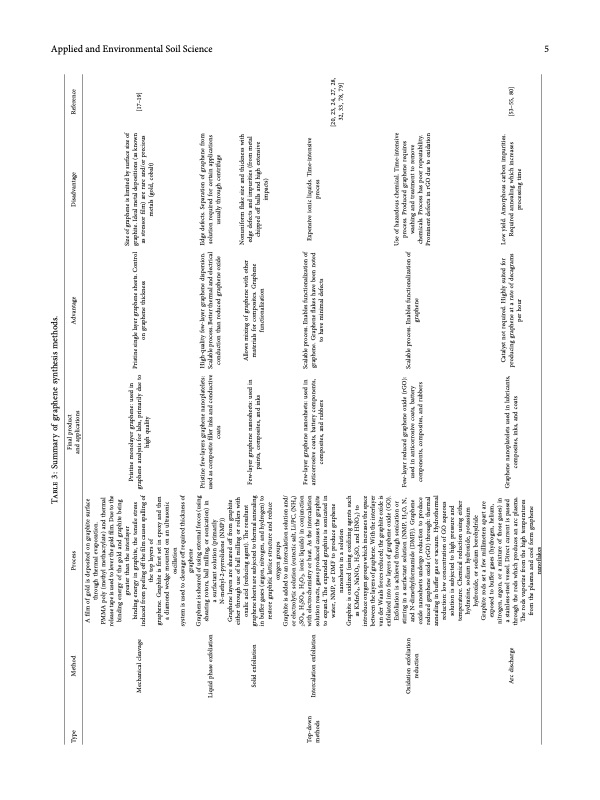
Applied and Environmental Soil Science 5 Type Method Mechanical cleavage Liquid phase exfoliation Solid exfoliation Intercalation exfoliation Oxidation exfoliation reduction Arc discharge Process Table 3: Summary of graphene synthesis methods. Final product and applications Advantage Disadvantage Size of graphene is limited by surface size of graphite. Ideal metal depositions (as known as stressor flm) are rare and/or precious metals (gold, cobalt) Reference [17–19] Top-down methods Few-layer graphene nanosheets: used in Scalable process. Enables functionalization of anticorrosive coats, battery components, graphene. Graphene fakes have been noted composites, and rubbers to have minimal defects A flm of gold is deposited on graphite surface through thermal evaporation. PMMA poly (methyl methacrylate) and thermal release tape is used to lever the gold flm. Due to the binding energy of the gold and graphite being greater than the interlayer binding energy in graphite, the tensile stress induced from peeling of the flm causes spalling of the top layers of graphene. Graphite is frst set in epoxy and then a diamond wedge mounted on an ultrasonic oscillation system is used to cleavage of required thickness of graphene Graphene is sheared of using external forces (using shearing rotors, ball milling, or sonication) in a surfactant solution (primarily N-methyl-2-pyrrolidone (NMP)) Graphene layers are sheared of from graphite either through use of ball milling or rollers with oxalic acid (reducing agent). Te resultant graphene sheets are subjected to thermal annealing in bufer gases (argon, nitrogen, and hydrogen) to restore graphitic lattice structure and reduce oxygen groups Graphite is added to an intercalation solution and/ or electrolytic solution (eutectic salt, Li/PC, (NH4) 2SO4, H2SO4, H2O2, ionic liquids) in conjunction with electrochemistry or heat. As the intercalation solution reacts, gases produced causes the graphite to expand. Te expanded graphite is sonicated in water, NMP, or DMF to produce graphene nanosheets in a solution Graphite is oxidized (using oxidizing agents such as KMnO4, NaNO3, H2SO, and HNO3) to introduce oxygen groups which increases the space between the layers of graphene. With the interlayer van der Waals forces reduced; the graphite oxide is exfoliated into few layers of graphene oxide (GO). Exfoliation is achieved through sonication or stirring in a surfactant solution (NMP, H2O, N, and N-dimethylformamide (DMF)). Graphene oxide nanosheets undergo reduction to produce reduced graphene oxide (rGO) through: thermal annealing in bufer gas or vacuum. Hydrothermal reduction: low concentration of GO aqueous solution is subjected to high pressure and temperature. Chemical reduction using either hydrazine, sodium hydroxide, potassium hydroxide, or sodium borohydride Graphite rods set a few millimeters apart are exposed to bufer gases (hydrogen, helium, nitrogen, argon, or a mixture of those gases) in a stainless-steel vessel. Direct current is passed through the rods which produces an arc plasma. Te rods vaporize from the high temperatures from the plasma and cool form graphene nanofakes Pristine monolayer graphene: used in graphene analysis for labs, primarily due to high quality Pristine single layer graphene sheets. Control on graphene thickness Pristine few-layers graphene nanoplatelets: High-quality few-layer graphene dispersion. Edge defects. Separation of graphene from used as composite fller inks and conductive Scalable process. Better thermal and electrical solution required for certain applications coats conduction than reduced graphene oxide usually through centrifuge Few-layer graphene nanosheets: used in paints, composites, and inks Allows mixing of graphene with other materials for composites. Graphene functionalization Nonuniform fake size and thickness with edge defects and impurities (from metal chipped of balls and high extensive impacts) Expensive ionic liquids. Time-intensive process Use of hazardous chemical. Time-intensive process. Produced graphene requires washing and treatment to remove chemicals. Process has poor repeatability. Prominent defects in rGO due to oxidation Low yield. Amorphous carbon impurities. Required annealing which increases processing time Few-layer reduced graphene oxide (rGO): used in anticorrosive coats, battery components, composites, and rubbers Graphene nanoplatelets used in lubricants, composites, inks, and coats Scalable process. Enables functionalization of graphene Catalyst not required. Highly suited for producing graphene at a rate of decagrams per hour [53–55, 80] [20, 23, 24, 27, 28, 32, 33, 78, 79]PDF Image | State-of-the-Art Graphene Synthesis Methods
PDF Search Title:
State-of-the-Art Graphene Synthesis MethodsOriginal File Name Searched:
8475504.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com (Standard Web Page)