
PDF Publication Title:
Text from PDF Page: 005
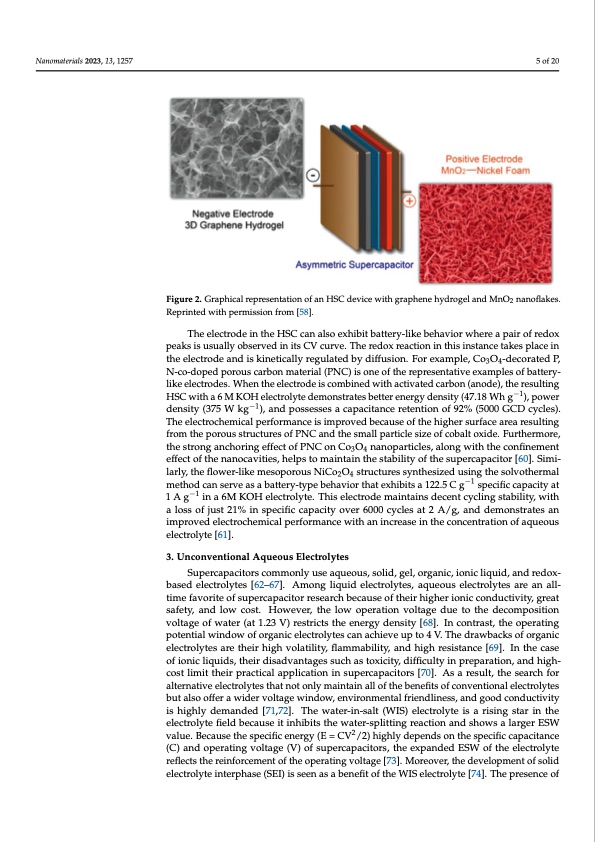
Naannoomaatteerriaialsls22002233,,1133,,1x25F7OR PEER REVIEW 5 of 2202 Fiigurre2.2G.rGaprahpichailcraelprespernetsaetniotantiofnanoHfSaCndHevSiCcewdeitvhicgerawphitehneghryadprhoegnelahnyddMrongOel2nanandoflMakneOs.2 nanoflakes. Reprinted with permission from [58]. Reprinted with permission from [58]. TAheheylbercitdrodeviincethceoHmSpCrisciangaglsroapehxhenibeitobxaytnteirtryi-dlieke(GbOehNa)vieolrecwtrhoedresaepxahiirboitfsreadcoax- −1 peackitsainscuesoufal7ly83o.5bsFergveadt ainciutsrrCeVntcduernvsei.tyThoefr1edAoxgre.aTchtieonGiOnNthiesleinctsrtoadncees tarkeepsrpoldacueceind the electrode and is kinetically regulated by diffusion. For example, Co O -decorated P, over a low-temperature hydrothermal route with an ammonia solution.3Th4is preparation N-co-doped porous carbon material (PNC) is one of the representative examples of battery- method results in the formation of quaternary, pyrrolic, pyridinic, and pyri- like electrodes. When the electrode is combined with activated carbon (anode), the resulting dinic-N-oxides on graphene oxide (GO), which not only improves the capacitance owing HSC with a 6 M KOH electrolyte demonstrates better energy density (47.18 Wh g−1), power to their positive charges but also enhances the electron transfer within the GO. A density (375 W kg−1), and possesses a capacitance retention of 92% (5000 GCD cycles). GON-based HSC exhibits a good cyclability of 5000 cycles with an 87% efficiency in The electrochemical performance is improved because o−f1 the higher surface area resulting−1 PVA/KOH gel electrolytes and produces a 40 Wh kg energy density at a 900 W kg from the porous structures of PNC and the small particle size of cobalt oxide. Furthermore, power density [59]. thestronganchoringeffectofPNConCoO nanoparticles,alongwiththeconfinement 34 The electrode in the HSC can also exhibit battery-like behavior where a pair of redox effect of the nanocavities, helps to maintain the stability of the supercapacitor [60]. Simi- peaks is usually observed in its CV curve. The redox reaction in this instance takes place larly,theflower-likemesoporousNiCoO structuressynthesizedusingthesolvothermal 24 in the electrode and is kinetically regulated by diffusion. For example, Co3O4-decorated method can serve as a battery-type behavior that exhibits a 122.5 C g−1 specific capacity at P, N-co-doped porous carbon material (PNC) is one of the representative examples of 1 A g−1 in a 6M KOH electrolyte. This electrode maintains decent cycling stability, with battery-like electrodes. When the electrode is combined with activated carbon (anode), a loss of just 21% in specific capacity over 6000 cycles at 2 A/g, and demonstrates an the resulting HSC with a 6 M KOH electrolyte demonstrates better energy density (47.18 improved electrochemical performance with an increase in the concentration of aqueous Wh g−1), power density (375 W kg−1), and possesses a capacitance retention of 92% (5000 electrolyte [61]. GCD cycles). The electrochemical performance is improved because of the higher surface area resulting from the porous structures of PNC and the small particle size of cobalt 3. Unconventional Aqueous Electrolytes oxide. Furthermore, the strong anchoring effect of PNC on Co3O4 nanoparticles, along Supercapacitors commonly use aqueous, solid, gel, organic, ionic liquid, and redox- with the confinement effect of the nanocavities, helps to maintain the stability of the su- based electrolytes [62–67]. Among liquid electrolytes, aqueous electrolytes are an all- percapacitor [60]. Similarly, the flower-like mesoporous NiCo2O4 structures synthesized time favorite of supercapacitor research because of their higher ionic conductivity, great using the solvothermal method can serve as a battery-type behavior that exhibits a 122.5 safety, and low cost. However, the low operation voltage due to the decomposition C g−1 specific capacity at 1 A g−1 in a 6M KOH electrolyte. This electrode maintains decent voltage of water (at 1.23 V) restricts the energy density [68]. In contrast, the operating cycling stability, with a loss of just 21% in specific capacity over 6000 cycles at 2 A/g, and potential window of organic electrolytes can achieve up to 4 V. The drawbacks of organic demonstrates an improved electrochemical performance with an increase in the concen- electrolytes are their high volatility, flammability, and high resistance [69]. In the case tration of aqueous electrolyte [61]. of ionic liquids, their disadvantages such as toxicity, difficulty in preparation, and high- cost limit their practical application in supercapacitors [70]. As a result, the search for 3. Unconventional Aqueous Electrolytes alternative electrolytes that not only maintain all of the benefits of conventional electrolytes Supercapacitors commonly use aqueous, solid, gel, organic, ionic liquid, and re- but also offer a wider voltage window, environmental friendliness, and good conductivity dox-based electrolytes [62–67]. Among liquid electrolytes, aqueous electrolytes are an is highly demanded [71,72]. The water-in-salt (WIS) electrolyte is a rising star in the all-time favorite of supercapacitor research because of their higher ionic conductivity, electrolyte field because it inhibits the water-splitting reaction and shows a larger ESW 2 great safety, and low cost. However, the low operation voltage due to the decomposition value. Because the specific energy (E = CV /2) highly depends on the specific capacitance voltage of water (at 1.23 V) restricts the energy density [68]. In contrast, the operating (C) and operating voltage (V) of supercapacitors, the expanded ESW of the electrolyte rpeoflteecntstitahlewrienindfowrceomf oerngtaonfitcheleocpteroralytitnegs vcaonltagchei[e7v3e].uMpotroe4ovVe.r,Tthedreavwelboapcmksenotf ofrgsoalnidic ellecttrrollytteesinatreerptheaisreh(SigEhI)vioslsaeteilnityas, flaabmenmeafibtioliftyth,eanWdIhSieglhecrtersoilsyttaen[c7e4[]6.9T]h.eInptrheesecnacseoff ionic liquids, their disadvantages such as toxicity, difficulty in preparation, and high-costPDF Image | Water-in-Salt Eutectic Solvent-Based Liquid Electrolytes
PDF Search Title:
Water-in-Salt Eutectic Solvent-Based Liquid ElectrolytesOriginal File Name Searched:
nanomaterials-13-01257.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |