
PDF Publication Title:
Text from PDF Page: 010
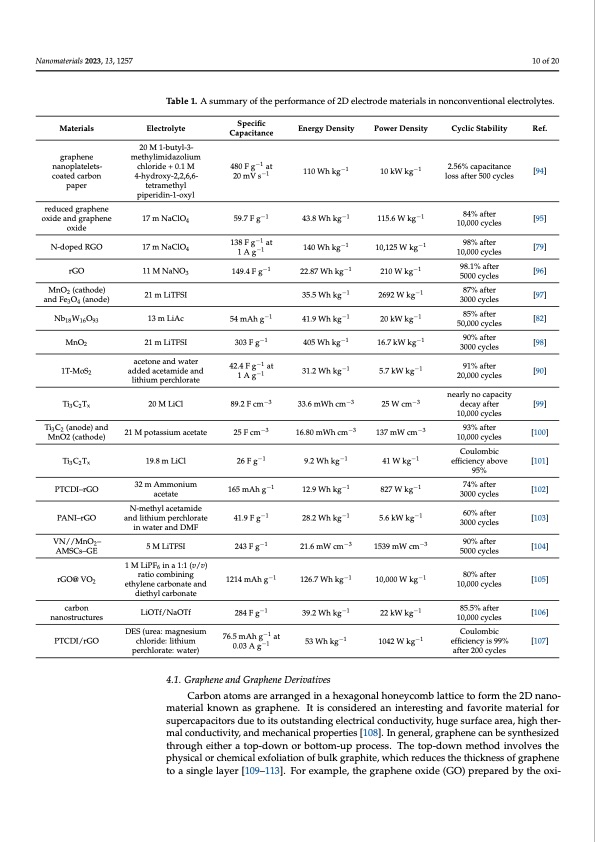
Nanomaterials 2023, 13, 1257 10 of 20 Table 1. A summary of the performance of 2D electrode materials in nonconventional electrolytes. Materials graphene nanoplatelets- coated carbon paper reduced graphene oxide and graphene oxide N-doped RGO rGO MnO2 (cathode) and Fe3O4 (anode) Nb18W16O93 MnO2 1T-MoS2 Ti3C2Tx Ti3C2 (anode) and MnO2 (cathode) Ti3C2Tx PTCDI–rGO PANI–rGO VN//MnO2– AMSCs–GE rGO@ VO2 carbon nanostructures PTCDI/rGO Electrolyte 20 M 1-butyl-3- methylimidazolium chloride + 0.1 M 4-hydroxy-2,2,6,6- tetramethyl piperidin-1-oxyl 17 m NaClO4 17 m NaClO 4 11 M NaNO3 21 m LiTFSI 13 m LiAc 21 m LiTFSI acetone and water added acetamide and lithium perchlorate 20 M LiCl 21 M potassium acetate 19.8 m LiCl 32 m Ammonium acetate N-methyl acetamide and lithium perchlorate in water and DMF 5 M LiTFSI 1 M LiPF6 in a 1:1 (v/v) ratio combining ethylene carbonate and diethyl carbonate LiOTf/NaOTf Specific Capacitance 480 F g−1 at 20 mV s−1 1 A g−1 149.4 F g−1 54 mAh g−1 303 F g−1 42.4 F g−1 at 1 A g−1 89.2 F cm−3 25 F cm−3 26 F g−1 165 mAh g−1 1214 mAh g−1 284 F g−1 Cyclic Stability Ref. 2.56% capacitance [94] loss after 500 cycles 84% after 10,000 cycles [95] 98% after [79] 10,000 cycles 98.1% after [96] 5000 cycles 87% after [97] 3000 cycles 85% after [82] 50,000 cycles 90% after [98] 3000 cycles 91% after 20,000 cycles [90] nearly no capacity decay after [99] 10,000 cycles 93% after [100] 10,000 cycles Coulombic efficiency above [101] 95% 74% after [102] 3000 cycles 60% after 3000 cycles [103] 90% after [104] 5000 cycles 80% after [105] 10,000 cycles 85.5% after [106] 10,000 cycles Coulombic efficiency is 99% [107] after 200 cycles Energy Density 110 Wh kg−1 −1 22.87 Wh kg−1 35.5 Wh kg−1 41.9 Wh kg−1 405 Wh kg−1 −1 33.6 mWh cm−3 16.80 mWh cm−3 9.2 Wh kg−1 12.9 Wh kg−1 −1 126.7 Wh kg−1 39.2 Wh kg−1 53 Wh kg−1 4.1. Graphene and Graphene Derivatives Power Density 10 kW kg−1 −1 210 W kg−1 2692 W kg−1 20 kW kg−1 16.7 kW kg−1 −1 59.7 F g 138 F g−1 at 43.8 Wh kg 140 Wh kg−1 115.6 W kg 10,125 W kg−1 −1 41.9 F g 243 F g−1 28.2 Wh kg 21.6 mW cm−3 5.6 kW kg 1539 mW cm−3 31.2 Wh kg 5.7 kW kg −1 25 W cm−3 137 mW cm−3 41 W kg−1 827 W kg−1 −1 10,000 W kg−1 22 kW kg−1 1042 W kg−1 DES (urea: magnesium chloride: lithium perchlorate: water) 76.5 mAh g−1 at 0.03 A g−1 Carbon atoms are arranged in a hexagonal honeycomb lattice to form the 2D nano- material known as graphene. It is considered an interesting and favorite material for supercapacitors due to its outstanding electrical conductivity, huge surface area, high ther- mal conductivity, and mechanical properties [108]. In general, graphene can be synthesized through either a top-down or bottom-up process. The top-down method involves the physical or chemical exfoliation of bulk graphite, which reduces the thickness of graphene to a single layer [109–113]. For example, the graphene oxide (GO) prepared by the oxi-PDF Image | Water-in-Salt Eutectic Solvent-Based Liquid Electrolytes
PDF Search Title:
Water-in-Salt Eutectic Solvent-Based Liquid ElectrolytesOriginal File Name Searched:
nanomaterials-13-01257.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |