
PDF Publication Title:
Text from PDF Page: 012
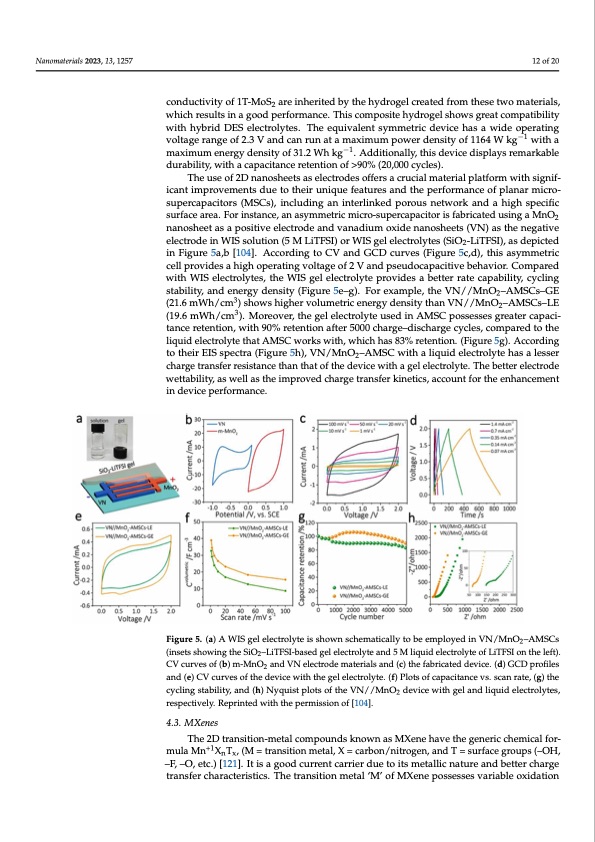
Nanomaterials 2023, 13, 1257 12 of 20 conductivity of 1T-MoS2 are inherited by the hydrogel created from these two materials, which results in a good performance. This composite hydrogel shows great compatibility with hybrid DES electrolytes. The equivalent symmetric device has a wide operating Nanomaterials 2023, 13, x FOR PEER REVIEW −1 13 of 22 voltage range of 2.3 V and can run at a maximum power density of 1164 W kg with a maximum energy density of 31.2 Wh kg−1. Additionally, this device displays remarkable durability, with a capacitance retention of >90% (20,000 cycles). Theuseoff22Dnnaannoosshheeetstsaasseleelcetcrtoroddesesofoffferesrsaacrcurcuiacilaml matearteiarliapllaptlfaotrfomrmwiwthitshigsnigif- inciafinctanimtpimropvreomvemntesndtsudeutoethoetihreuirniuqnuiequfeafteuarteusreasndantdhethperpfoerfmoramncaencoefpoflapnlarnmaricmroi- supercapacitors (MSCs), including an interlinked porous network and a high specific cro-supercapacitors (MSCs), including an interlinked porous network and a high specific surface area. For instance, an asymmetric micro-supercapacitor is fabricated using a MnO surface area. For instance, an asymmetric micro-supercapacitor is fabricated using a2 nanosheet as a positive electrode and vanadium oxide nanosheets (VN) as the negative MnO2 nanosheet as a positive electrode and vanadium oxide nanosheets (VN) as the electrode in WIS solution (5 M LiTFSI) or WIS gel electrolytes (SiO -LiTFSI), as depicted negative electrode in WIS solution (5 M LiTFSI) or WIS gel electrol2ytes (SiO2- LiTFSI), as in Figure 5a,b [104]. According to CV and GCD curves (Figure 5c,d), this asymmetric depicted in Figure 5a,b [104]. According to CV and GCD curves (Figure 5 c-d), this cell provides a high operating voltage of 2 V and pseudocapacitive behavior. Compared asymmetric cell provides a high operating voltage of 2 V and pseudocapacitive behavior. with WIS electrolytes, the WIS gel electrolyte provides a better rate capability, cycling Compared with WIS electrolytes, the WIS gel electrolyte provides a better rate capability, stability, and energy density (Figure 5e–g). For example, the VN//MnO –AMSCs–GE 2 cycling stability, and energy density (Figure 5e–g). For example, the VN//MnO2–AMSCs– (21.6mWh/cm3)sh3owshighervolumetricenergydensitythanVN//MnO–AMSCs–LE 2 GE (21.6 mWh/cm ) shows higher volumetric energy density than VN//MnO2–AMSCs– (19.6 mWh/cm3). Moreover, the gel electrolyte used in AMSC possesses greater capaci- LE (19.6 mWh/cm3). Moreover, the gel electrolyte used in AMSC possesses greater ca- tance retention, with 90% retention after 5000 charge–discharge cycles, compared to the pacitance retention, with 90% retention after 5000 charge–discharge cycles, compared to liquid electrolyte that AMSC works with, which has 83% retention. (Figure 5g). According the liquid electrolyte that AMSC works with, which has 83% retention. (Figure 5 g). Ac- to their EIS spectra (Figure 5h), VN/MnO2–AMSC with a liquid electrolyte has a lesser cording to their EIS spectra (Figure 5h), VN/MnO2–AMSC with a liquid electrolyte has a charge transfer resistance than that of the device with a gel electrolyte. The better electrode lesser charge transfer resistance than that of the device with a gel electrolyte. The better wettability, as well as the improved charge transfer kinetics, account for the enhancement electrode wettability, as well as the improved charge transfer kinetics, account for the in device performance. enhancement in device performance. Figure 5. (a) A WIS gel electrolyte is shown schematically to be employed in VN/MnO2–AMSCs Figure 5. (a) A WIS gel electrolyte is shown schematically to be employed in VN/MnO2–AMSCs (insets showing the SiO2–LiTFSI-based gel electrolyte and 5 M liquid electrolyte of LiTFSI on the (insets showing the SiO2–LiTFSI-based gel electrolyte and 5 M liquid electrolyte of LiTFSI on the left). left). CV curves of (b) m-MnO2 and VN electrode materials and (c) the fabricated device. (d) GCD CV curves of (b) m-MnO2 and VN electrode materials and (c) the fabricated device. (d) GCD profiles profiles and (e) CV curves of the device with the gel electrolyte. (f) Plots of capacitance vs. scan rate, and (e) CV curves of the device with the gel electrolyte. (f) Plots of capacitance vs. scan rate, (g) the (g) the cycling stability, and (h) Nyquist plots of the VN//MnO2 device with gel and liquid electro- cycling stability, and (h) Nyquist plots of the VN//MnO2 device with gel and liquid electrolytes, lytes, respectively. Reprinted with the permission of [104]. respectively. Reprinted with the permission of [104]. 4.3. MXenes 4.3. MXenes The 2D transition-metal compounds known as MXene have the generic chemical The 2D transition-metal compounds known as MXene have the generic chemical for- formula M+1n+1XnTx, (M = transition metal, X = carbon/nitrogen, and T = surface groups (– mula Mn XnTx, (M = transition metal, X = carbon/nitrogen, and T = surface groups (–OH, OH, –F, –O, etc.) [121]. It is a good current carrier due to its metallic nature and better –F, –O, etc.) [121]. It is a good current carrier due to its metallic nature and better charge charge transfer characteristics. The transition metal ‘M’ of MXene possesses variable ox- transfer characteristics. The transition metal ‘M’ of MXene possesses variable oxidation idation numbers and some of the MXenes exhibit semiconducting behavior. MXenes have shown great performances as high-rate electrodes for pseudocapacitors due to their highly reversible redox reactions and electrical conductivity. Generally, the MXenes are synthesized using (i) top-down and (ii) bottom-up methods. In the top-down approach, the methods are an etching process by HF acid/fluoride salt, alkalis, molten salt, electro-PDF Image | Water-in-Salt Eutectic Solvent-Based Liquid Electrolytes
PDF Search Title:
Water-in-Salt Eutectic Solvent-Based Liquid ElectrolytesOriginal File Name Searched:
nanomaterials-13-01257.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |