PDF Publication Title:
Text from PDF Page: 014
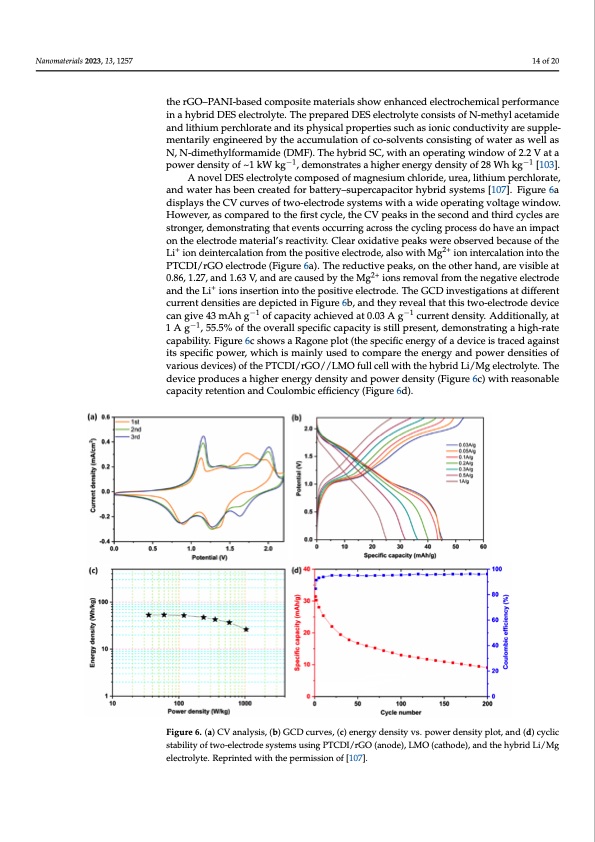
Nanomaterials 2023, 13, 1257 14 of 20 the rGO–PANI-based composite materials show enhanced electrochemical performance in a hybrid DES electrolyte. The prepared DES electrolyte consists of N-methyl acetamide Nanomaterials 2023, 13, x FOR PEER REVIEW 15 of 22 and lithium perchlorate and its physical properties such as ionic conductivity are supple- mentarily engineered by the accumulation of co-solvents consisting of water as well as N, N-dimethylformamide (DMF). The hybrid SC, with an operating window of 2.2 V at a N, N-dimethylformamide (−D1MF). The hybrid SC, with an operating window of 2.2 −V1at a power density of ~1 kW kg , demonstrates a higher energy density of 28 Wh kg [103]. power density of ⁓1 kW k g−1, demonstrates a higher energy density of 28 Wh kg−1 [103]. A novel DES electrolyte composed of magnesium chloride, urea, lithium perchlorate, A novel DES electrolyte composed of magnesium chloride, urea, lithium perchlo- and water has been created for battery–supercapacitor hybrid systems [107]. Figure 6a rate, and water has been created for battery–supercapacitor hybrid systems [107]. Figure displays the CV curves of two-electrode systems with a wide operating voltage window. 6a displays the CV curves of two-electrode systems with a wide operating voltage win- However, as compared to the first cycle, the CV peaks in the second and third cycles are dow. However, as compared to the first cycle, the CV peaks in the second and third cycles stronger, demonstrating that events occurring across the cycling process do have an impact are stronger, demonstrating that events occurring across the cycling process do have an on the electrode material’s reactivity. Clear oxidative peaks were observed because of the impact on the electrode material’s reactivity. Clear oxidative peaks were observed be- Li+ ion deintercalation from the positive electrode, also with Mg2+ ion intercalation into the cause of the Li+ ion deintercalation from the positive electrode, also with Mg2+ ion inter- PTCDI/rGO electrode (Figure 6a). The reductive peaks, on the other hand, are visible at calation into the PTCDI/rGO electrode (Figure 6a). The reductive peaks, on the other 0.86, 1.27, and 1.63 V, and are caused by the Mg2+ ions removal from the negative electrode hand, are visible at 0.86, 1.27, and 1.63 V, and are caused by the Mg2+ ions removal from and the Li+ ions insertion into the positive electrode. The GCD investigations at different the negative electrode and the Li+ ions insertion into the positive electrode. The GCD in- current densities are depicted in Figure 6b, and they reveal that this two-electrode device vestigations at different current densities are depicted in Figure 6b, and they reveal that can give 43 mAh g−1 of capacity achieved at 0.03 A g−1 current density. Additionally, at this two-electrode device can give 43 mAh g−1 of capacity achieved at 0.03 A g−1 current 1 A g−1, 55.5% of the overall specific capacity is still present, demonstrating a high-rate density. Additionally, at 1 A g−1, 55.5% of the overall specific capacity is still present, capability. Figure 6c shows a Ragone plot (the specific energy of a device is traced against demonstrating a high-rate capability. Figure 6c shows a Ragone plot (the specific energy its specific power, which is mainly used to compare the energy and power densities of of a device is traced against its specific power, which is mainly used to compare the en- various devices) of the PTCDI/rGO//LMO full cell with the hybrid Li/Mg electrolyte. The ergy and power densities of various devices) of the PTCDI/rGO//LMO full cell with the device produces a higher energy density and power density (Figure 6c) with reasonable hybrid Li/Mg electrolyte. The device produces a higher energy density and power den- capacity retention and Coulombic efficiency (Figure 6d). sity (Figure 6c) with reasonable capacity retention and Coulombic efficiency (Figure 6d). Figure 6. (a) CV analysis, (b) GCD curves, (c) energy density vs. power density plot, and (d) cyclic Figure 6. (a) CV analysis, (b) GCD curves, (c) energy density vs. power density plot, and (d) cyclic stability of two-electrode systems using PTCDI/rGO (anode), LMO (cathode), and the hybrid Li/Mg stability of two-electrode systems using PTCDI/rGO (anode), LMO (cathode), and the hybrid Li/Mg electrolyte. Reprinted with the permission of [107]. electrolyte. Reprinted with the permission of [107]. 5. Conclusion and Future Perspectives The faster depletion of fossil fuels and their environmental impact has prompted the research community to seek alternate and viable sources of energy. Electrochemical SCsPDF Image | Water-in-Salt Eutectic Solvent-Based Liquid Electrolytes
PDF Search Title:
Water-in-Salt Eutectic Solvent-Based Liquid ElectrolytesOriginal File Name Searched:
nanomaterials-13-01257.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com (Standard Web Page)