
PDF Publication Title:
Text from PDF Page: 229
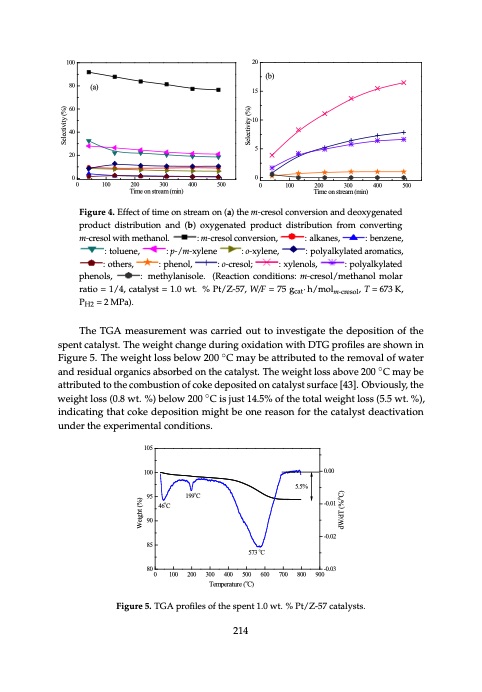
60650 700 707007070 550 600 650 700 750 )uer(eK()K) erearaat(utKurere)e(K(K)) TemTpemraptuereat(uKre) (K) TeTemeTmpTepemrmeaprptpaeueetruareaarteu(tuKr(ereK)e()K(K)) Cadtaealycstitvsa2ti0o1n6i,s6attributedtotheformationofoxygenatedcompoundswhichmayleadtotheformationof 9 CaCtaCalaCtyastsaltystslasy2tlssy0ts12t6260s,0,1261606,1,66, 6 coke on catalysts. The variation of the alkanes, o-xylene, and polyalkylated benzene’s selectivities is 4 4 insignificant. Benzene, phenol, methylanisole, and other products are in minor amounts and their deactivation is attributed to the formation of oExyEcgEexepxcEntecxapeacatptlelektkpdatlanckleaokaslnamk,neapsean,sore,ousam,nraordaomtstrimiocacwmstsai,htcaiiscatac,isnch,dsam,napndpahdhnyepdnphleohepalnehidnocesosltni,olcoiscolt,ishtc,hesoe,otfrhotoherptremhroepadrdprtuoirpococdrtntduosucdotcsusftucsctsshusucsahacushcahisansda selectivities change slightly with increasing the time on stream. npdahnedpnhopelnhicoesnlci,iocolsoik,ctehsoe,totrhonetprhcreoaprdtrauoplcdyrtousdctstus.ucTtcshuhcesahusvchaisrsniadaisatnindoienan,ndeaon,nfaepnt,hatpepnhhaptlhlehkantalhenad,dneleasrd,ni,idevedaorea,adnin-rtvetidixdahvarnyieteaevditlistsvherai,ventaisieihevlrl,ses,e,aoiosrala,senloasxdoliesoxpetxioiesnilxtsytisisasnmitlnksaiamnsyllmllsamlamtmllealaodolmalumbanonmetoutsnun.o.zntusetn.sn.tse.’s selectivities is , and phenolliics,, ottherr prroductts such as iindane,, naphtthallene,, and ttheiirr maomunotusn.ts. 5 mallmllaomuonoutusn.nttsisn..signif1i0c0ant. Benzene, phenol, methylanisole, and o2t0her products are in minor amounts and their 100101000100 selectivities5ch5an5geslightlywithincreasingthetime(oa)n)(as()at)r(bae)am. 80 (b) (b)((b)) 5 55 5 (b) (b()b)(b) (a) 100 4 4 (b) 80 808080 60 40 20 1 0 0 60 60 (b) 60 60 10 15 40 404040 40 3 0 750 7570570750 100 200 300 400 500 3 3 (a)3 3 4 4 4 4 4 3 333 434 15 20 80 60 40 20 alsank,neaesn,s,e,s, : b:e:nb:zbeenbnzeezen,enznee,n,e, : to:l:tuto:otelolnutuloeuel,nenunee,n,e, : p::-p:/pmp-:/p--/-mpxm/-my-/-xlmx-eyxyn-lyxlelynenleneene : o-:xo:oyo-:lxlx-eoyxyn-ylxel,y,nelne,e,n,e, : p:op:lpyopo:alolyplyklkoayaylakylklayakytlelylekadlytaeltadertdoeamdraoraomtrimiocacamstsati,i,tcaiscst,i,sc,s, : o:th:oeot:rhtsohe,trehsre,sr,s, : p allkanes,, ::beFenizgzeunre,,4.:Epf-f/e:m:ct-toxoollyufletninme,,eonst:r:e:oap-m--x//myol--nexny(yelale,e)ntehem-c::r:epsoolylacloknyvlaetresdionaraonmdadtiecos,xygenat:edopthreordsu,ct alylyklakaytleylklkadlyaytleltlaedartdteoeadmdaroraoamtrrimocoamsamti,itacaiactstti,s,ic,s,, : o:th:oe:ot:rhotsohoe,thetrhsre,s,er,rs,, : p:h:ep:n:p:h:ohpeplenh,hnoeonln,lo,ol,, : o:-c:or:o-e-:c-socro-er-clcecs;rosoreols;slo;oll;; : x:y:lx:e:xyxnylyxoellylneslno,n,eolnoslo,lss,l,s, : p::o:plpy:opoalolplyklkoyaylaylklakytleylelkadlytateltadepdtdheepdnphoheplneshsno.eolns(l(o.sR.lse(.Ra(Rce(taeRiocaentcnaitocionctinon distributi:onphaendol,(b) oxy:geon-acrtedsol;product :dixstyrliebnuotliso,n from :copnovleyratliknyglatmed-crpehsoenlolws,ith 2 2 2 2 2 222 1 111 0 000 550 555050550 2 15 1 1 1 20 2020120 0 000 000 550 55505505050 600 610600600 T T e e m m Tp p e e e T r mr a a e t p t mTu u e r e r p r e e ma e t ( r ( p u Ka K e r t ) u e ) r a r ( e t Ku ( r ) Ke ) ( K ) 600 606000600 T T 550550 50 600600 650650 60 650 700 700700 70 750 750750 750 600Timeonstre6a5m0 (min) 550 00 0 100 200 300 400 500 0 100 200 300 400 500 menta-iacoctrtnentieiimosotnonenplmetterepcameoturpnparevetetruerarortesetunuirorore(enenao)o(nanptnh)(r(de(aoatah)dc)edotutthehnhcoeveotoxencycdrovosoginenisneorvtnvsnreiaeirorbtrasensunidiodotaninodpdanearnondaxdeunyodcgdxeteoyonxg(gxdxbayeyiyt)ngsgegtaederoetninexbpadayurtteoetgepidoedruononpcdartrouotaodedcdndidtususdudtctcrcrdiptistibdtrsruotirstbditibourouiuntnbcitoutiaontndinodainsa(tndbardi)n)(bdo(oub)x(t)ybyoiogogx)nexeyonygxfageyrtneoegenadmdetaneptpaderctdoeopdpnruorvpcocdertduorducdtdticiunstdtctgrditisibdtsrutirstbitibouroiutnbitouifonrtrionofmrnforofmrmo-mcmrmr-ecm-srcoe-rlcse distribution and (b) oxygenated product distribution from converting m-cresol with Time on stream (min) Time on stream (min) ngdgaeiestnetraditbepudrtoipodrnuocfdrtuodcmitsdcdtroiisnbtvrurietbmirout-intnciorogfenrmsoofm-rlcowrmesi-tocmhmlr-e-mwcsiroetehtlshHomalnDeHotOhlDa.nOo.l. :m:-cm:rrem-e-scs-orcoelrlsecosolnclvoceoencrnvosveinioroevsnrneis,oriosnin,o,n,:al:lk:ala:nkleaekaslsnak,neasen,se,s,:b:e:nbzb:eenebnzezn,nezne,en,e,:to:l:tuot:eolnutloeuel,neune,en,e xgxyeyngaetneadattepdropdrrouodcdtucdctitsdmtdriisisesbtruhriibtbabiunuoutotntiiolo.fnrnofmfrrom-m:mcmr-m-e-cs-rorcelrseoHoslloDHlODcD.oOn..versi:omn:,:-mcmr-e-csrcroreoelsnso:ovllearlskiaone,s, ::albkeanzeesn,e, :be:ntzoelnue,ne, :toluene, : p:ol:pyoaplykoaylyllkaytlekladytleadpdtehdepnhopeplnhso.elns(o.o.Rllses(,RaR, cetaiocntioncn:o:ncmcdomientetidhotihntytiyisolola:nanspi:isop=ple.=2(R2M2eaPMcat,PWPioan/n,W,FW/c=FW/oF7n=/F5d=7ig=75tci5ao7gt·n5gchacst/ga·m:thc·amh/otm·/lh-mccor/ermolsecolrolsce,rsoleoHcslro,/e2ls,H/omclH,2re/eH2ct/srch2oe/ralcsenro=seolos5l=lo,=lmc5=a5,ot,c5alacal,ytarcatsalatytl=aysltsy1t=s.=0t1=1w.0.1t0.w.0w%t.wt.%Pt%t./P%ZPt-/t5ZP/7Zt- : p::oplpyoallykayllklkaytlelladattedpdhpepnhoenlnso.olls(.R. (e((RaRcetaiacoctntiioncnoncodonintdidoiitntiioso:nsp:: p=p =2 2M2 WPMa/P,PFPa=,, 75 gcat·h/molcresol, H2/cresol = 5, catalyst = 1.0 wt. % Pt/Z-57 methanol:. methy:lamni-scorles.ol(Rceoancvtieornsiocno,nditions:: amlk-acnreso,l/metha:nboelnzmeonlea,r ratio :=tolu1e/4n,e, 2e/Htch,r2aHe/cnsro/eclsr=oemls5o=,lac5=ra,t5cara,lytciasaolttya=slrty1a=s.1t0i/1o=1w.,0=1tp.w01%=t/w. 4P%t2,.t/%cPZPMat-/t5PZa7tal-/)y5Z,.s7W-t5)./7=F).1=.0 7w5t.gca%t·hP/mt/oZlc-re5s7ol, W/F = 75 g ̈ h/mol , T = 673 K, essooll,,2H22//crresoll = 5,, cattallystt = 1..0 wtt.. % Ptt//Z--57)).. cat m-cresol = 1.0 wt. % Pt/Z-57).P = 2 MPa). H2 GeGnGerGneanelellrelnyare,la,lrlytatlhy,lle,ytht,cheatehtcaeaclytyacatailaicyctlaytiHlHtcyicDtHicOHDHDOoDOf oOofxfoyofgogxexeyonygx-geycnegon-enc-ntoca-onicntoaitininantiganinicncngoignmcgocpomcomoupumnpnododupsusnondudsnsdu catalyst = 1.0 wt. % Pt/Z-57, W/F = 75 gcat·h/molm-cresol, T = 673 K, PH2 = 2 MPa). : p-/m-xylene : o-xylene, : polyalkylated aromatics, : others, : phenol, : o-cresol; : xylenols, : polyalkylated phenols, ODOof ofxyogxeyng-ecno-nctoanintainingincgomcopmoupnodusndusnduenrdedrifdfdeifrffefenrtenctoncdoindtidiofidntftiedisofrifndnefuefsinsrefteifrunenecsgrntieiantntcagtaclytacatsalatytslaysltsiyststiksinsiokskwnnoknownwotnowntnpotorotpocpreorpeocrdecoectdeehdertodhtuhrgotrhohurugogthuwhgothwtwrotewoarocertaeirocaetcnaitociopntinaotpnhpawtaphtahw DHODOoOfoffxoyoxgxyeyng-ecno--ncotoanitnttaiiningiincgomcopmopuponoudunsndusnudunenrdedrrifdfdeiifrfefenrrtenctotncdcodoiofninftdeidoiriitentiinosotnuscsaiunutsagsilinyngsgts is known to proceed through two reaction pathwa The TGA measurement was carried out to investigate the deposition of the spent catalyst. The weight : methylanisole. (Reaction conditions: m-cresol/methanol molar ratio = 1/4, wcntntpnoivrtotitopotocyreopeopcrdfreo7ectd8ehe.ere2dtotdh%hurtgtothdhurogotuhupwugsgohth1hw1rtot.tew3wa%orocoetaicrorceoentamiacocptptntiaiaotrnpnhneawdtphawawytithsthahwyw[stsa3ahy4ya[s,s3t34w4[5[[3,3]3i34.t45h,,3T]o3.5u5h5]t]TeT].m.hoTehTehnhtyheoedheanrhrynpoeoydoahngndrntlypeohre(dnanog8rtpopgeiph8hosalanea.gyt2tnothieshsh%sosliniyslioiysisolisysrisdodioirsirdeoedcicrrtitedrdceieretceotdcxdetyoedgogxeexeyonygxageytneigionoaentanitoa(i(onDtinoD(Dn(OD()DOoDO)fO)opofh)feponphfoheplnehsnoetolonoslosptplortrsoptdopruorpcocdreduoucadecruoeacmr change during oxidation with DTG profiles are shown in Figure 5. The weight loss below 200 °C may The TGA measurement was carried out to investigate the deposition of the oyornxgxexayeaytngsgigoeaesntniaoat(aotDtntiioDo(3nDn.O6(D(%D)DOoDf)a)OtcOpoa)h)f7)teo2opaonf3fhlfoypepKlsnhshtoe.et=nlnonTsop1htlolsrsi.so0tpttdororwueopcsdtdr.uerouol%dcacdtdereuiocPmcacmertepo/oaZlamtrirrieo-ocoa5smth7itacay,hatdtahWhiicrtyco/hdhthFchyryayoeodr=dcbrarao7ocrcnct5baisaovor.grbnbinbTtcosesoeoyaoh.ntnon·niTeTosnheso.hfheo/.ntTitmTethTshteihoheseohoetleiehteulrstchmhoeoeoetet-nthutcrhcherepoceslorlurocereuplod,pluTelrpdein=lndergdi6rnis7ngrai3gtntsugaKsrtaus,tauiriPoatruHtnanir2t/oairon=atainp/por2/ianrdpa/MrpidadiePpdhdiadyde)d.dhedrhryeaydhtdiriyoardntanirt/oaihontyin/odh/nrhryo/ydhgdryeordnogrageotneigonaoetnanitoai(ontHinoY(YnH(D be attributed to the removal of water and residual organics absorbed on the catalyst. The weight loss spent catalyst. The weight change during oxidation with DTG profiles are shown in uruntrerir/aoasratnsnantieipo/ordinand/p/rbrridiayadpephidmidydeedhdtryehaehdhathiyrynyoadodntrlir/a.oahatntyCiio/odohonrnym/o//hdhgpyryeoaodndrgrearoeodtngigoaewentniiaoat(tnhnHtiioY(cnHnoD(Y-Y(H)fHeDYtYeYo)dDitptno)o)r)gotptodormuopcdedrertouohdcdcdaeuynucoceTlTeylyo,hchcpcTlelyteyayohTchcrcpsesalhleTeloeaofoecbrhfrpspaiaoeaeansafnrcerfsfrsadadzoici.aenfeonfcfspspfnidio.inanaedntstspsh.d.pa. taiphntahvitonhilnlvivoneolsvsvlovetlhshevesethstihentetheienerirnmtmeitnremtrdemieramdetedeieaidtpapeitraeoptdepruorpcocdrtduoucdtycyutclyctoychchlcyeolxcxohlaheonxehoxaelnaxnoaolrnlosorlurbos above 200 °C may be attributed to the combusti ̋on of coke deposited on catalyst surface [43]. Obviously, The TGA measurement was carried out to investigate the deposition of the spent catalyst. The weight t.e0inr7mt%erermdtioeadt0ei.a0pte%eropdaruotocd5tu7c3ytcKclyocahlneodxhaefnxroalmnolr13os.ur8b%ssutbitsouttie1tud1t.e5cdy%clyaocthle7ox2ha3esnxtoKauaslnd).tso,oyutIlus,dwn.tdytuhIhty,dnhie,ltyierhst,ehetitirehishsesreriuseinsduisuentnduedenctetdeatecebtcleatecbatblaelmbealoemaumanomotuunontuftnomotffeomtfmheymtelhtcehytylhclylycolychlcyeolcxohlaheonxehoxaelnaxnoianolnloitnhilneitnhtphertehopedpr thienetieinrtmterermdieadtieiiatpteropdrrouodcdtuctytclyochlloeoxhaenxoanlnolrlosorurbsusutbibtsuttititetudttecdyclyochlloeosxhthuaednxyoan,ln.otoIhlln.e.IrItInenhniitstshiiusndetectable amount of methylcyclohexanol in the prod Figure 5. The weight loss below 200 C may be attributed to the removal of water the weight loss (0.8 wt. %) below 200 °C is just 14.5% of the total weight loss (5.5 wt. ̋%), indicating change during oxidation with DTG profiles are shown in Figure 5. The weight loss below 200 °C may o(mufrnotumnotf9o.om8f%emthteyotlhc1y5lc.c1lyo%chleoaxhtae5nx7oa3lnoiKnl aitnhnedthfperropmdruo3cd9tus.c,c9t%ism, tpiomly4pi2nly.g9in%tghatrthteata7rhtce2retaet3tihrocaDeKetcnaiDto)ciDrDo.nOotiDnDTuortohnOreouisrumtoetueamtiemnalmiaynianloyilcnycoluyocrcosuccudrcsrusrdridusnurgdirnuitnghrigenthgtmhet-ehmcemr-ecm-srcoe-rlcseroHseolDslHoOHlDHDpODOropOcpreorpsocsrecoseucsnesdusuensrndudetnhrederteh emaomuonoutunontft omffemtheytthlhcyllclyochlloeoxhaenxoanlnoionll itinhnetthperopdrrouodcdtusc,cttsism,, ipimlypilnlygiinrtghgeattcthtatiahtottentthrDeoeDuDtOeDmOainly occurs during the m-cresol HDO process under the and residual organics absorbed on the catalyst. The weight loss above 200 C may be that coke deposition might be one reason for the catalyst deactivation under the experimental conditions. ersunusmdrgrdiunuetrhgtrihientyghghmgleat-ththcmhmiebeore-e-enmcsmroa-e-clctfcstrHorbeilsesDbsoHoHnouOlzlDtHeHeHpnOdDreDoOptOtcororeopsptcrshroreoeluscucsneredsueunsemnesru.duotneTnhvrdhdeaetehlrtrremtotsththtfetsdswhteyecasdldsotattentcecrdidodoiancnctcniodonidintfdidsoritiiteotn(tiWpsilosouind/(=(/neWFspsunsW2(e/(a=(pFWp/lpMcF27=7=o/=o=FP5=MurM2ag72lg=g,d7P5PaMcM5na7bgtP·,·Pi5ePcghchaca/st/g,a·,m,thc·aho/otbm·/lhmscor/oemolsrcolbrolce,resleoHcdslro,e2ls,/Ho/oclHn,2r/eH2cts/shrc2oe/relcsero=sceolas5l=to)a=l.l5T=yT)5.sh)h5t.Te.)Trh.eTeheTfhreohererefeorfw,eorafereol,eikr,gaealahn,nklteaekaslsnlakoneiansnsensitetnhisnetihnptpherteohpdepruorpcocd ̋ indicating that coke deposition might be one reason for the catalyst deactivation attributed to the combustion of coke deposited on catalyst surface [43]. Obviously, the weightloss(0.8wt.%10)5below200 Cisjust14.5%ofthetotalweightloss(5.5wt.%), roe/eclxsr=oyels5eo=n)l.e5=Ts).hs5eaTeT)lb.rheoTcefvtrorhierevferio2,etyr0faeo,l0,ikr.ea°e,lCn.k,aeatlmshnkeieansnpye-tih/nsnbmeit-nhpaxertyohtplprdeirbnuopecudrtotusedcdmltuesctacomtmytsivtabmhiyeteaybgyc(eoabngmerbnangnaxueterirnshmadteytitredueoedhfadrdrmhryntoeydhfmogdodryefeofrfdntomgmc2rhragoeo4oetnemtkti.gih0honoae%entantnihdtoaoioeontfipnotonofslftuoiotefeolnutdloeue.lo.neune.enc.ea.talyst surface [43]. Obviously, 22//crresoll = 5)).. Therrefforre,, allkanes iin tthe prroductts may be generratted ffrrom tthe as that withthouetwmeeitghhatnolols(sa (m0a.8xiwmtu.m%o)fb1e6l.o7w%2a0t069°8CKis).jMusotr1eo4v.5e%r, thoef the total weight loss (5.5 wt. %), indicating oyoxlxeynlloenlnsoallsnsdanmndemtheytthlhaynlliasnoniliseso[lle3e1[[3]3;1(]2];;)((2m2))emtheytthlhaytlillaoatntiionfnobo1feff9nb9bzeCenzentwnoeiwgtwhiivitmtthehetmtohleauttnheh 100 2.22.2.C2.2.a.Ct2taC.alaCtyataiailcytlatyHiltcyiyctHdicHryoHdydryeordodrxdeoyoedgoxeyxongyxageytneginoaoeantnitoaiaontninodananMndadenMtdMheyMtelhataehyttilyihoaolyatniltoaoiontfinomomnof-fCmComfr-eCem-sCsr-oerClserosevolvselorovlePvPoretv/r/PeZ s also another reaction to produce p-/m-xylene. Similarly, the further that coke deposition might be one reason for the catalyst deactivation under the experimental conditions. under the experimental conditions. oantioandanMdeMtheytlhaytiloantionf mof-Cmr-eCsroelsovleorvPert/PZt-t/5Z7-57 gaentniaoatntiioanadanMdeMtheyttlhaytlilaoatntiionf omoff-mCm-r--CeCsroelsolvlleorvePert/PZPt-t/5/Z7--57 5.5% takes place which can be demonstrated by the increa ding methanol (i.e., from 2.8% without meth4a6nCol to 5.3% with methanol ngioaentniaoatniodanMdaneMdtheMytlhaeytihloayntliaoatntiomant-Cmamtr-emCs-roCels/rMoels/eoMtlh/eMatnheoatlnhMoanloMllaoMrlRaoralaRtRiroaRtoiaofti1o/f1o1f/1/1 ygenattiion and Metthyllattiion att m--Crresoll//Metthanoll Mollarr Rattiio off 1//1 95 105 2.2.1. Catalytic hydrodeoxygenation and Methylation at m-Cresol/Methan o -0.01 100 0.00 se of polyalkylated isaormu-uicsoserumtehseyomtlhaeysttielhloalynetilicoaortetniavorcienetaiairoecenstaisocoaotnrniefsosrnoesfbfarorsceftarcncveteasdcnottafrsrniontsmrteioinr0mtie.en1ertm%deireamadtenieasadtmeie0asa.t9emys%oamyctcaouycr0(co1a.uc0)s)r(rc%m1mf(auao1)sre(la)mlaf1ftonohomhs)ewdlyfmlteololhs0tal:wewhy.lt4toiyilshoa%wl:yntanisltoa:oiontfinococrnrofefcosrcoferlcseirosneoltsiolonilxntoiytnolxteoxynylxoelylneslnoeaolnsnlodsdalnasmndaednmthdmheymtelhtaehyntylhiaslsynaolnilaseinos[oil3selo1e[l]3[e;31([1]23;])1;(m2]m(;2)e()m2thmh)eymtelhtaehy varriiousmetthyllattiionrreacttiionsoffrreacttanttsorriintterrmediiattesmayoccurrasffolllows:: 5.5% ey3nl.oe3eln%soaltnsoda1nm.d1e%mtheyatlnhadynli1as.no6il%seo[la3et1[7]3;21(3]2;)K(m2.)Temtheyrtlhehaytailroaentisoenfvboeoerfanblzerenen 95 o 85 onanleno[ol3ll6]; (3) methylation of toluene producing xylenes (o-, m- a o 4 4 750 757050750 FigFuigreur4e. E4.ffEefcfteocft otifmteimoen ostnresatmreaomn (oan) t(hae) Ftmhig-geFcuFirmrgreiFegeu-sciuorg1rlereu.ecsr1Eoe1.fnlf.fE1vceE.foecfrtEnefsocveofifotcfetnrorcrseoeftaiafonorcendrftaeidroacoaetncnaiotodcitxonetediynmotgenptpoemenxtmrepayampetgteureupeadrnetaeuprtauroteotrenuedor(u(eonacn)ot(nat(tha)(e)athtc)cheotehncevoceoencrnvosveiniorevsnrneisoriaosninoda cat cresol 2 62500656050650 300700 707000740 Timeonstream(min) 0.00 o 199 C 22.2.2.1.21. .C1C.ataCatalaytlaytiltcyicthichyydhdryordodrdeooedoxexyoygxgeynegnaetanitoaiontinoanandadnMdMeMtehtehytylhalytailtoaiontinoantatmamt-Cm-Cr-erCseroseols/lMo/Ml/eMteh 90 ByBcByoyB-cfocyeo-ecf-deofie-nfdegedienmidngeigmnthmgeamtenhtoehaltna,hnovaolan,lrvo,ivaol,rauvirsoaioumriusoesmuthmseymtelhtaehyttiylhoalytnailtoraieontainrocerntaeiorcaetnciatsocionotinsfosroneofsafrcoertfaearcanetctastncaotntasrtnsiontorstrieonri By co-feeding methanol, various methylation reactions of reactants or inter -0.02 zaesnonweneistwthofiomtgthrotieovtmgthtehogievastitgtovnehrileoeavatuolsnteoulonutlloleute:lne[un3ee6n[]3[e;36([6]3;]6);(]3m(;3)e()m3tmh)eymtelhtaehyttylihaolytaniltoaioontfinotonoflftuotefolnutloeuelnepunereeonpdperuorpocdriduonucdgicunixcngyignlxegxynylxelsynelne(eosen-s(e,o(smo-(,-om,a 573 C o -0.01 ieloarntsioinfntoflcutoreelnuseoenplseroapdnrudocdxiunycglienxngyolxlesynlvleisna(eoasn-(,oam-l,k-myaln-adati4npo6d-nCxpry-elxaeycnltei)onn[e3,)0r[,e3s(074p,–)3e3(3c47p7(t94)–o–i(])v3lp34;yep9o9)alo]l]ynply;lk;doyaylalanylknldadakydtlylekadlytaebltadetdnbezdbeenebnzeznsnezneaesensdaenaspndoadnlpydpoaolplylkoyaylaylklakytlyelkadlytaepltadehtdependphoheplnehsnoefolnrslosfmlrfsortofmhrmoetmhmthetemhtmheeymtelhtaehyttylihaolytan thlhaytlillaoatntiionf otoffltutoellnuenpnereopdrrouodcdiuncgiinxgyxlxeynllesne(eos-(,(om--,-,ma-n--danpd-xpy--xlxeynlle)ne[e3))(0[4[[3,3)30p7,,o3–3l737y–9–a3]3l3;k9ay]]n;l;adatnend benzenes and polyalkylated phenols from the methylation 80 -0.03 daornlpgpydoaplyHkoaylDlylkaOytleklaidyntlepatdhthepednhpoperlnhseoesfnlersonfmlcrseoftmrhooeftmthmetehmteheyamtnlhaeoytlihl.oayntAt9li0aotnsnf0imoxonyaflox1le0fyn0axlemeysn2olae0u0nsnndeatsnx3o0day0fmnlxedpeyeynmo4txloh0mhely0eylananmstleanohoetwloeonhlal5ks0tlnoaiy0h,twnolhlarsaolein,twtls6seoh,r0pdie0lrte,esehcrpset7peis0vce0petceiltlvcyiy8vet.0ile0vyley. l.9y0.0 andpollyallkyllattedphenollsffrromtthemetthyllattiionoffxyllenesandxyllenollswiitth Weight (%) Weight (%) dW/dT (%/oC) dW/dT (%/oC) Selectivity (%) Selectivity (%) Selectivity (%) Selectivity(%) Selectivity (%) Selectivity (%) Selectivity (%) Sellecttiiviitty(%) Selectivity (%SS)eellectivity((%)) Selectivity (%) Selectivity (%) Sellecttiiviitty(%) Selectivity (%) Selectivity (%) Selectivity (%) attioioiof o1of/f1)//1i1s))piisrsepsrerenstendtteaeadsds asfauauanffucutnicoctntiionf otoeffmttepmerpaetrurartetur(r(resese(e(seFeigFuiigrgeur2r2re)e).2)).. Temperature (oC) observed compared with negligible amount in the absence of methanol, CaCtaCalytyaCatailaicyctlaythiltcyiyctdhirchryoydhdryeordodrxdeoyoedgogxeexeyonygxageytneigionaoetnanitoaiaontnindodanamndaedenmtdhmheymtelhtaehyttiylihoaolytnaniltoaiontfinomnof-fcmcormrf-ecm-srcoe-rlcsewrowseoleslwrorewelecwrecearaercraeciera caondamryetmhyeltahtyiolantiofnmo-fcxryesleonlowlse.re carrie8d5 out to examine the possible iaotnioandanmdemtheythlaytliaotnioonfomf-mcr-ecsroelsowlewrercearcraierrdieoduotutot teoxaemxaimneifnftehfeceftftehefpcefeoftcepfsftcsoitimsmofbsfloeiembtfthmhleamtenhtoeohatlnahnoaolnlolonthnoetnhthestehlseescletesilvevcelteceitvcivectioeovncevoceoencrnvosveinorevsnrneisoriosnininotiononintopiptno-t/pomp--/-xmpx/my-y-/lxm-exyn-ylxel.y.nelneT. nattiion and metthyllattiion off m--crresoll werre carrriied outt tto examiine tthe possiiblle Figure 5. TGA profiles of the spent 1.0 wt. % Pt/Z-57 catalysts. 573 oC heseseoecfltseismevceletetleietcvchctctetaioivnvnecoevolencicrnovosonentinhorvseneieroHrrsnisiDiniotOnoinotiopinfn-tmt/topmpo--c-/pxrmpe-y-/-/s/lmxomeyln--xlxbexey.rynilnleTe.gnheseTeT.e.hvTpeThrhoaepeldrnuopepcdrwtrouodcdrdtueuicascdtcttritmisibdotd-rurincmicsistsbsrmtrtit-re,reourcim-sisinbtbrcnboioieu-uoroculcset/t/n(ltirmoimsuaioeoltdnes/ne(lnmiota/atnhmhlteg(/(a(amtaenanhttoeohatlnahmnomaolnolmolmalormolrarlaoratlririaoartaoiortoafifoto1iof/1f1o)1/f1i/s1)/p)pi1sri)espsisprsernpsetresenedsnteatndestdeadsafsuauanasnfcuaftunifoncountcnitocoiontfinotenofmftoeptpfmetmrep 80 -0.03 u1e/n1e),isasprweseellnatesdxaysleanfeusnacntidonotohfetrecmopmepraotunreds(,sewehFicighuorebv2i)o.usly improve 0 100 200 300 400 500 600 700 800 900 ne by 44%, possibly through direct methylation of toluene ando HDO Temperature ( C) Figure 5. TGAprofifillessoffttheesspentt1.0.0wtt..%PPt/t/Z-57caattallysstts.. 214 -0.02PDF Image | Zeolite Catalysis
PDF Search Title:
Zeolite CatalysisOriginal File Name Searched:
Zeolite_Catalysis.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |