
PDF Publication Title:
Text from PDF Page: 047
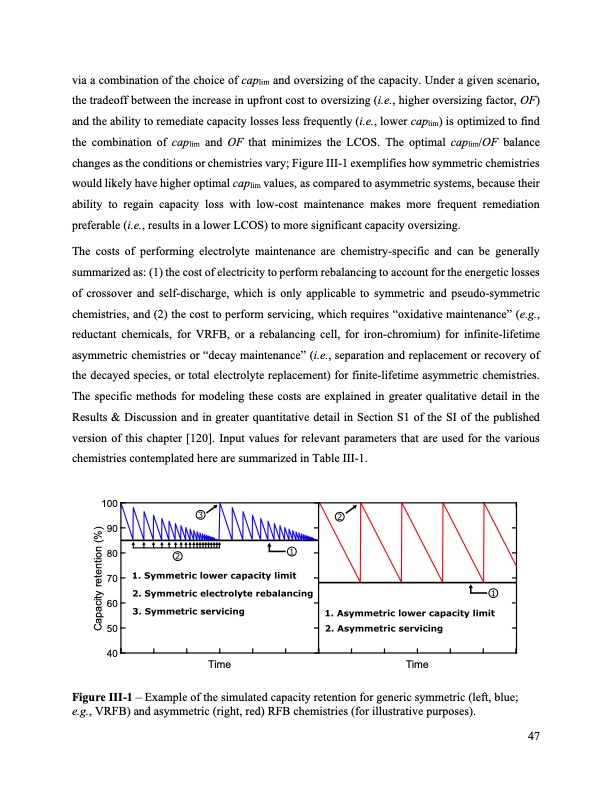
via a combination of the choice of caplim and oversizing of the capacity. Under a given scenario, the tradeoff between the increase in upfront cost to oversizing (i.e., higher oversizing factor, OF) and the ability to remediate capacity losses less frequently (i.e., lower caplim) is optimized to find the combination of caplim and OF that minimizes the LCOS. The optimal caplim/OF balance changes as the conditions or chemistries vary; Figure III-1 exemplifies how symmetric chemistries would likely have higher optimal caplim values, as compared to asymmetric systems, because their ability to regain capacity loss with low-cost maintenance makes more frequent remediation preferable (i.e., results in a lower LCOS) to more significant capacity oversizing. The costs of performing electrolyte maintenance are chemistry-specific and can be generally summarized as: (1) the cost of electricity to perform rebalancing to account for the energetic losses of crossover and self-discharge, which is only applicable to symmetric and pseudo-symmetric chemistries, and (2) the cost to perform servicing, which requires “oxidative maintenance” (e.g., reductant chemicals, for VRFB, or a rebalancing cell, for iron-chromium) for infinite-lifetime asymmetric chemistries or “decay maintenance” (i.e., separation and replacement or recovery of the decayed species, or total electrolyte replacement) for finite-lifetime asymmetric chemistries. The specific methods for modeling these costs are explained in greater qualitative detail in the Results & Discussion and in greater quantitative detail in Section S1 of the SI of the published version of this chapter [120]. Input values for relevant parameters that are used for the various chemistries contemplated here are summarized in Table III-1. 100 90 80 70 60 50 40 Time Time Capacity retention (%) Figure III-1 – Example of the simulated capacity retention for generic symmetric (left, blue; e.g., VRFB) and asymmetric (right, red) RFB chemistries (for illustrative purposes). 47PDF Image | Bringing Redox Flow Batteries to the Grid
PDF Search Title:
Bringing Redox Flow Batteries to the GridOriginal File Name Searched:
Rodby-krodby-phd-chemE-2022-thesis.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |