
PDF Publication Title:
Text from PDF Page: 026
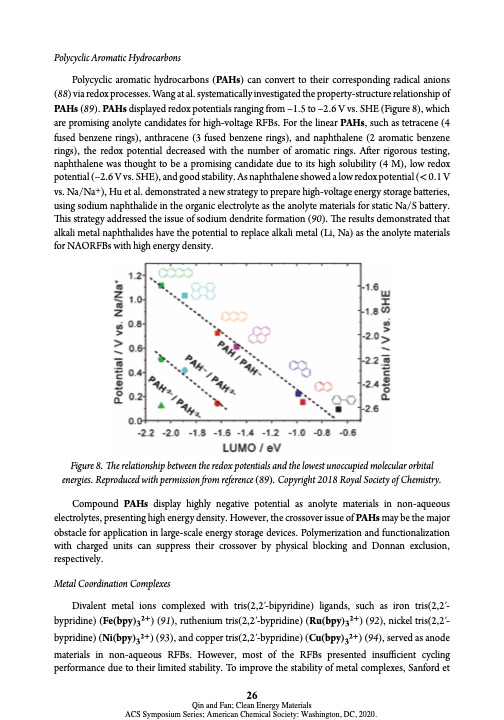
Polycyclic Aromatic Hydrocarbons Polycyclic aromatic hydrocarbons (PAHs) can convert to their corresponding radical anions (88) via redox processes. Wang at al. systematically investigated the property-structure relationship of PAHs (89). PAHs displayed redox potentials ranging from –1.5 to –2.6 V vs. SHE (Figure 8), which are promising anolyte candidates for high-voltage RFBs. For the linear PAHs, such as tetracene (4 fused benzene rings), anthracene (3 fused benzene rings), and naphthalene (2 aromatic benzene rings), the redox potential decreased with the number of aromatic rings. After rigorous testing, naphthalene was thought to be a promising candidate due to its high solubility (4 M), low redox potential (–2.6 V vs. SHE), and good stability. As naphthalene showed a low redox potential (< 0.1 V vs. Na/Na+), Hu et al. demonstrated a new strategy to prepare high-voltage energy storage batteries, using sodium naphthalide in the organic electrolyte as the anolyte materials for static Na/S battery. This strategy addressed the issue of sodium dendrite formation (90). The results demonstrated that alkali metal naphthalides have the potential to replace alkali metal (Li, Na) as the anolyte materials for NAORFBs with high energy density. Figure 8. The relationship between the redox potentials and the lowest unoccupied molecular orbital energies. Reproduced with permission from reference (89). Copyright 2018 Royal Society of Chemistry. Compound PAHs display highly negative potential as anolyte materials in non-aqueous electrolytes, presenting high energy density. However, the crossover issue of PAHs may be the major obstacle for application in large-scale energy storage devices. Polymerization and functionalization with charged units can suppress their crossover by physical blocking and Donnan exclusion, respectively. Metal Coordination Complexes Divalent metal ions complexed with tris(2,2′-bipyridine) ligands, such as iron tris(2,2′- bypridine) (Fe(bpy)32+) (91), ruthenium tris(2,2′-bypridine) (Ru(bpy)32+) (92), nickel tris(2,2′- bypridine) (Ni(bpy)32+) (93), and copper tris(2,2′-bypridine) (Cu(bpy)32+) (94), served as anode materials in non-aqueous RFBs. However, most of the RFBs presented insufficient cycling performance due to their limited stability. To improve the stability of metal complexes, Sanford et 26 Qin and Fan; Clean Energy Materials ACS Symposium Series; American Chemical Society: Washington, DC, 2020.PDF Image | Electroactive Materials Next-Generation Redox Flow Batteries
PDF Search Title:
Electroactive Materials Next-Generation Redox Flow BatteriesOriginal File Name Searched:
bk-2020-1364.ch001.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |