
PDF Publication Title:
Text from PDF Page: 006
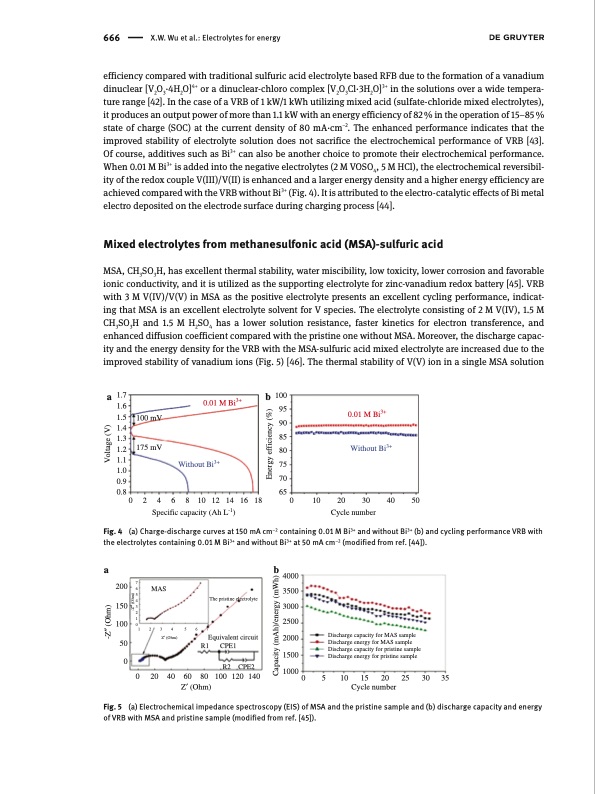
666 X.W. Wu et al.: Electrolytes for energy efficiency compared with traditional sulfuric acid electrolyte based RFB due to the formation of a vanadium dinuclear [V2O3·4H2O]4+ or a dinuclear-chloro complex [V2O3Cl·3H2O]3+ in the solutions over a wide tempera- ture range [42]. In the case of a VRB of 1 kW/1 kWh utilizing mixed acid (sulfate-chloride mixed electrolytes), it produces an output power of more than 1.1 kW with an energy efficiency of 82 % in the operation of 15–85 % state of charge (SOC) at the current density of 80 mA·cm–2. The enhanced performance indicates that the improved stability of electrolyte solution does not sacrifice the electrochemical performance of VRB [43]. Of course, additives such as Bi3+ can also be another choice to promote their electrochemical performance. When 0.01 M Bi3+ is added into the negative electrolytes (2 M VOSO4, 5 M HCl), the electrochemical reversibil- ity of the redox couple V(III)/V(II) is enhanced and a larger energy density and a higher energy efficiency are achieved compared with the VRB without Bi3+ (Fig. 4). It is attributed to the electro-catalytic effects of Bi metal electro deposited on the electrode surface during charging process [44]. Mixed electrolytes from methanesulfonic acid (MSA)-sulfuric acid MSA, CH3SO3H, has excellent thermal stability, water miscibility, low toxicity, lower corrosion and favorable ionic conductivity, and it is utilized as the supporting electrolyte for zinc-vanadium redox battery [45]. VRB with 3 M V(IV)/V(V) in MSA as the positive electrolyte presents an excellent cycling performance, indicat- ing that MSA is an excellent electrolyte solvent for V species. The electrolyte consisting of 2 M V(IV), 1.5 M CH3SO3H and 1.5 M H2SO4 has a lower solution resistance, faster kinetics for electron transference, and enhanced diffusion coefficient compared with the pristine one without MSA. Moreover, the discharge capac- ity and the energy density for the VRB with the MSA-sulfuric acid mixed electrolyte are increased due to the improved stability of vanadium ions (Fig. 5) [46]. The thermal stability of V(V) ion in a single MSA solution a b 4000 3500 3000 2500 2000 a 1.7 1.6 1.5 1.4 1.3 1.2 1.1 1.0 0.9 0.8 0.01 M Bi3+ b 100 95 100 mV 90 85 175 mV 80 Without Bi3+ 75 70 0.01 M Bi3+ Without Bi3+ 0 2 4 6 8 10 12 14 16 18 Specific capacity (Ah L-1) 65 0 10 20 Cycle number Fig. 4 (a) Charge-discharge curves at 150 mA cm–2 containing 0.01 M Bi3+ and without Bi3+ (b) and cycling performance VRB with the electrolytes containing 0.01 M Bi3+ and without Bi3+ at 50 mA cm–2 (modified from ref. [44]). 30 40 50 200 76 150 MAS 5 4 3 2 1 The pristine electrolyte 100 0 1234567 50 0 Z′ (Ohm) Equivalent circuit R1 CPE1 Discharge capacity for MAS sample Discharge energy for MAS sample Discharge capacity for pristine sample R2 CPE2 0 20 40 60 80 100120140 Z′ (Ohm) 10000 5 10 15 20 25 30 35 Cycle number 1500 Discharge energy for pristine sample Fig. 5 (a) Electrochemical impedance spectroscopy (EIS) of MSA and the pristine sample and (b) discharge capacity and energy of VRB with MSA and pristine sample (modified from ref. [45]). -Z′′ (Ohm) Voltage (V) -Z′′ (Ohm) Capacity (mAh)/energy (mWh) Energy efficiency (%)PDF Image | Electrolytes for vanadium redox flow batteries
PDF Search Title:
Electrolytes for vanadium redox flow batteriesOriginal File Name Searched:
10-1515_pac-2013-1213.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |