
PDF Publication Title:
Text from PDF Page: 071
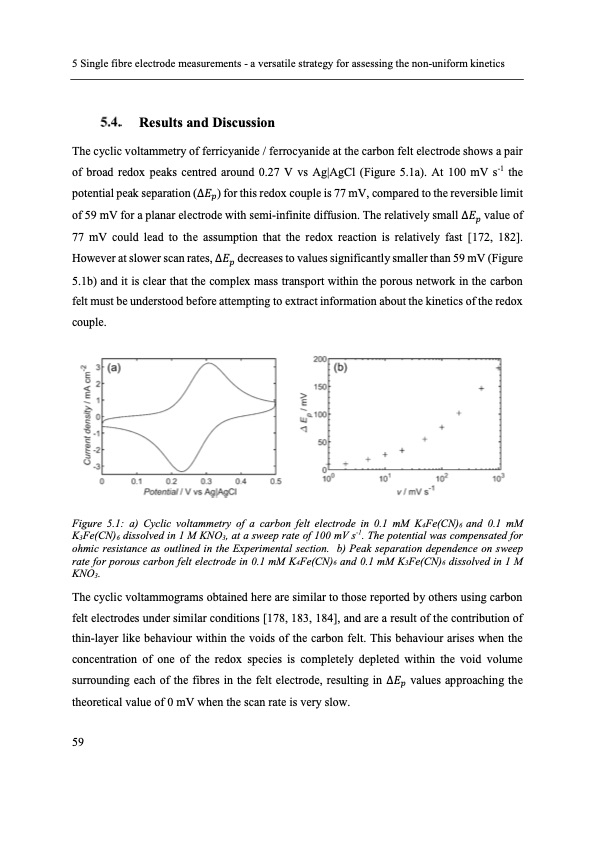
5 Single fibre electrode measurements - a versatile strategy for assessing the non-uniform kinetics Results and Discussion The cyclic voltammetry of ferricyanide / ferrocyanide at the carbon felt electrode shows a pair of broad redox peaks centred around 0.27 V vs Ag|AgCl (Figure 5.1a). At 100 mV s-1 the potential peak separation (Δ𝐸𝑝) for this redox couple is 77 mV, compared to the reversible limit of 59 mV for a planar electrode with semi-infinite diffusion. The relatively small ∆𝐸𝑝 value of 77 mV could lead to the assumption that the redox reaction is relatively fast [172, 182]. However at slower scan rates, Δ𝐸𝑝 decreases to values significantly smaller than 59 mV (Figure 5.1b) and it is clear that the complex mass transport within the porous network in the carbon felt must be understood before attempting to extract information about the kinetics of the redox couple. Figure 5.1: a) Cyclic voltammetry of a carbon felt electrode in 0.1 mM K4Fe(CN)6 and 0.1 mM K3Fe(CN)6 dissolved in 1 M KNO3, at a sweep rate of 100 mV s-1. The potential was compensated for ohmic resistance as outlined in the Experimental section. b) Peak separation dependence on sweep rate for porous carbon felt electrode in 0.1 mM K4Fe(CN)6 and 0.1 mM K3Fe(CN)6 dissolved in 1 M KNO3. The cyclic voltammograms obtained here are similar to those reported by others using carbon felt electrodes under similar conditions [178, 183, 184], and are a result of the contribution of thin-layer like behaviour within the voids of the carbon felt. This behaviour arises when the concentration of one of the redox species is completely depleted within the void volume surrounding each of the fibres in the felt electrode, resulting in Δ𝐸𝑝 values approaching the theoretical value of 0 mV when the scan rate is very slow. 59PDF Image | Electron Transfer Kinetics in Redox Flow Batteries
PDF Search Title:
Electron Transfer Kinetics in Redox Flow BatteriesOriginal File Name Searched:
electron-transfer-flow-batteries-thesis.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |