
PDF Publication Title:
Text from PDF Page: 112
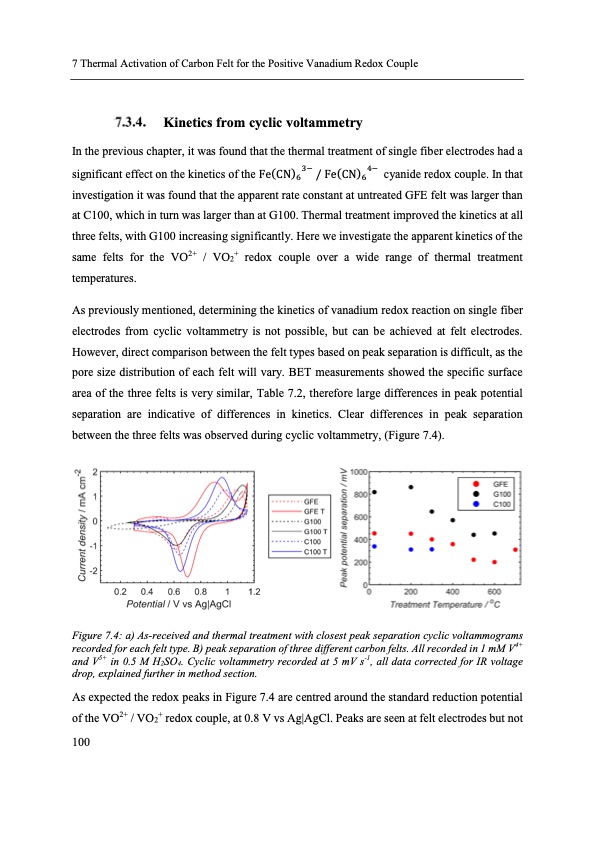
7 Thermal Activation of Carbon Felt for the Positive Vanadium Redox Couple Kinetics from cyclic voltammetry In the previous chapter, it was found that the thermal treatment of single fiber electrodes had a significant effect on the kinetics of the Fe(CN) 3− / Fe(CN) 4− cyanide redox couple. In that 66 investigation it was found that the apparent rate constant at untreated GFE felt was larger than at C100, which in turn was larger than at G100. Thermal treatment improved the kinetics at all three felts, with G100 increasing significantly. Here we investigate the apparent kinetics of the same felts for the VO2+ / VO2+ redox couple over a wide range of thermal treatment temperatures. As previously mentioned, determining the kinetics of vanadium redox reaction on single fiber electrodes from cyclic voltammetry is not possible, but can be achieved at felt electrodes. However, direct comparison between the felt types based on peak separation is difficult, as the pore size distribution of each felt will vary. BET measurements showed the specific surface area of the three felts is very similar, Table 7.2, therefore large differences in peak potential separation are indicative of differences in kinetics. Clear differences in peak separation between the three felts was observed during cyclic voltammetry, (Figure 7.4). Figure 7.4: a) As-received and thermal treatment with closest peak separation cyclic voltammograms recorded for each felt type. B) peak separation of three different carbon felts. All recorded in 1 mM V4+ and V5+ in 0.5 M H2SO4. Cyclic voltammetry recorded at 5 mV s-1, all data corrected for IR voltage drop, explained further in method section. As expected the redox peaks in Figure 7.4 are centred around the standard reduction potential of the VO2+ / VO2+ redox couple, at 0.8 V vs Ag|AgCl. Peaks are seen at felt electrodes but not 100PDF Image | Electron Transfer Kinetics in Redox Flow Batteries
PDF Search Title:
Electron Transfer Kinetics in Redox Flow BatteriesOriginal File Name Searched:
electron-transfer-flow-batteries-thesis.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |