
PDF Publication Title:
Text from PDF Page: 007
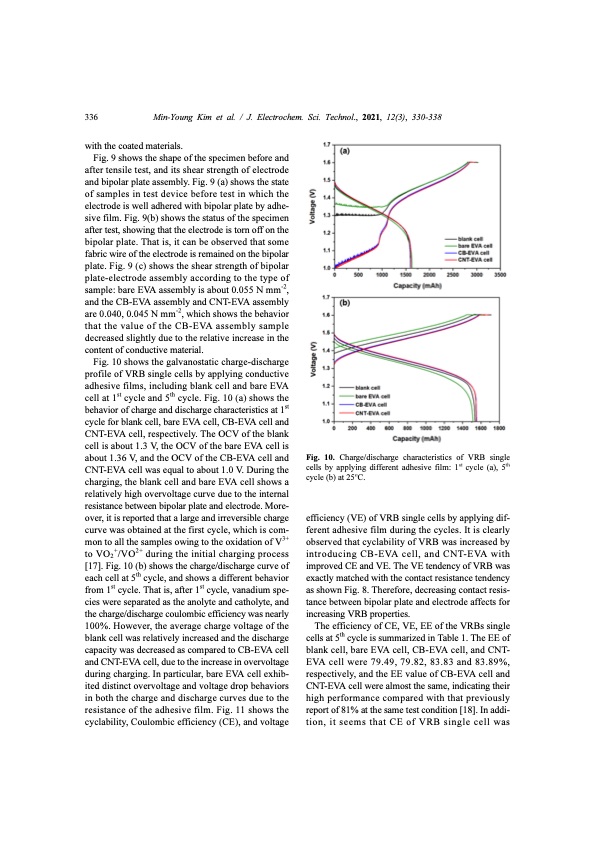
336 Min-Young Kim et al. / J. Electrochem. Sci. Technol., 2021, 12(3), 330-338 with the coated materials. Fig. 9 shows the shape of the specimen before and after tensile test, and its shear strength of electrode and bipolar plate assembly. Fig. 9 (a) shows the state of samples in test device before test in which the electrode is well adhered with bipolar plate by adhe- sive film. Fig. 9(b) shows the status of the specimen after test, showing that the electrode is torn off on the bipolar plate. That is, it can be observed that some fabric wire of the electrode is remained on the bipolar plate. Fig. 9 (c) shows the shear strength of bipolar plate-electrode assembly according to the type of sample: bare EVA assembly is about 0.055 N mm-2, and the CB-EVA assembly and CNT-EVA assembly are 0.040, 0.045 N mm-2, which shows the behavior that the value of the CB-EVA assembly sample decreased slightly due to the relative increase in the content of conductive material. Fig. 10 shows the galvanostatic charge-discharge profile of VRB single cells by applying conductive adhesive films, including blank cell and bare EVA cell at 1st cycle and 5th cycle. Fig. 10 (a) shows the behavior of charge and discharge characteristics at 1st cycle for blank cell, bare EVA cell, CB-EVA cell and CNT-EVA cell, respectively. The OCV of the blank cell is about 1.3 V, the OCV of the bare EVA cell is about 1.36 V, and the OCV of the CB-EVA cell and CNT-EVA cell was equal to about 1.0 V. During the charging, the blank cell and bare EVA cell shows a relatively high overvoltage curve due to the internal resistance between bipolar plate and electrode. More- over, it is reported that a large and irreversible charge curve was obtained at the first cycle, which is com- mon to all the samples owing to the oxidation of V3+ to VO2+/VO2+ during the initial charging process [17]. Fig. 10 (b) shows the charge/discharge curve of each cell at 5th cycle, and shows a different behavior from 1st cycle. That is, after 1st cycle, vanadium spe- cies were separated as the anolyte and catholyte, and the charge/discharge coulombic efficiency was nearly 100%. However, the average charge voltage of the blank cell was relatively increased and the discharge capacity was decreased as compared to CB-EVA cell and CNT-EVA cell, due to the increase in overvoltage during charging. In particular, bare EVA cell exhib- ited distinct overvoltage and voltage drop behaviors in both the charge and discharge curves due to the resistance of the adhesive film. Fig. 11 shows the cyclability, Coulombic efficiency (CE), and voltage efficiency (VE) of VRB single cells by applying dif- ferent adhesive film during the cycles. It is clearly observed that cyclability of VRB was increased by introducing CB-EVA cell, and CNT-EVA with improved CE and VE. The VE tendency of VRB was exactly matched with the contact resistance tendency as shown Fig. 8. Therefore, decreasing contact resis- tance between bipolar plate and electrode affects for increasing VRB properties. The efficiency of CE, VE, EE of the VRBs single cells at 5th cycle is summarized in Table 1. The EE of blank cell, bare EVA cell, CB-EVA cell, and CNT- EVA cell were 79.49, 79.82, 83.83 and 83.89%, respectively, and the EE value of CB-EVA cell and CNT-EVA cell were almost the same, indicating their high performance compared with that previously report of 81% at the same test condition [18]. In addi- tion, it seems that CE of VRB single cell was Fig. 10. Charge/discharge characteristics of VRB single cells by applying different adhesive film: 1 cycle (a), 5 cycle (b) at 25 C. ht ts oPDF Image | Energy Efficiency Improvement of Vanadium Redox Flow Battery
PDF Search Title:
Energy Efficiency Improvement of Vanadium Redox Flow BatteryOriginal File Name Searched:
jecst-2021-00017.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |