
PDF Publication Title:
Text from PDF Page: 002
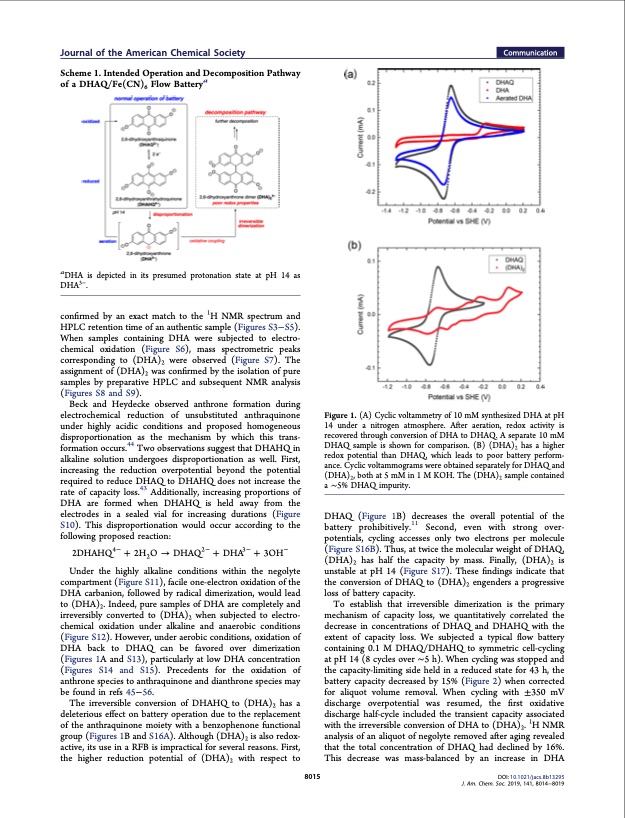
Journal of the American Chemical Society Communication Scheme 1. Intended Operation and Decomposition Pathway of a DHAQ/Fe(CN)6 Flow Batterya aDHA is depicted in its presumed protonation state at pH 14 as DHA3−. confirmed by an exact match to the 1H NMR spectrum and HPLC retention time of an authentic sample (Figures S3−S5). When samples containing DHA were subjected to electro- chemical oxidation (Figure S6), mass spectrometric peaks corresponding to (DHA)2 were observed (Figure S7). The assignment of (DHA)2 was confirmed by the isolation of pure samples by preparative HPLC and subsequent NMR analysis (Figures S8 and S9). Beck and Heydecke observed anthrone formation during electrochemical reduction of unsubstituted anthraquinone under highly acidic conditions and proposed homogeneous disproportionation as the mechanism by which this trans- formation occurs.44 Two observations suggest that DHAHQ in alkaline solution undergoes disproportionation as well. First, increasing the reduction overpotential beyond the potential required to reduce DHAQ to DHAHQ does not increase the rate of capacity loss.43 Additionally, increasing proportions of DHA are formed when DHAHQ is held away from the electrodes in a sealed vial for increasing durations (Figure S10). This disproportionation would occur according to the following proposed reaction: Figure 1. (A) Cyclic voltammetry of 10 mM synthesized DHA at pH 14 under a nitrogen atmosphere. After aeration, redox activity is recovered through conversion of DHA to DHAQ. A separate 10 mM DHAQ sample is shown for comparison. (B) (DHA)2 has a higher redox potential than DHAQ, which leads to poor battery perform- ance. Cyclic voltammograms were obtained separately for DHAQ and (DHA)2, both at 5 mM in 1 M KOH. The (DHA)2 sample contained a ∼5% DHAQ impurity. DHAQ (Figure 1B) decreases the overall potential of the battery prohibitively.11 Second, even with strong over- potentials, cycling accesses only two electrons per molecule (Figure S16B). Thus, at twice the molecular weight of DHAQ, (DHA)2 has half the capacity by mass. Finally, (DHA)2 is unstable at pH 14 (Figure S17). These findings indicate that the conversion of DHAQ to (DHA)2 engenders a progressive loss of battery capacity. To establish that irreversible dimerization is the primary mechanism of capacity loss, we quantitatively correlated the decrease in concentrations of DHAQ and DHAHQ with the extent of capacity loss. We subjected a typical flow battery containing 0.1 M DHAQ/DHAHQ to symmetric cell-cycling at pH 14 (8 cycles over ∼5 h). When cycling was stopped and the capacity-limiting side held in a reduced state for 43 h, the battery capacity decreased by 15% (Figure 2) when corrected for aliquot volume removal. When cycling with ±350 mV discharge overpotential was resumed, the first oxidative discharge half-cycle included the transient capacity associated with the irreversible conversion of DHA to (DHA)2. 1H NMR analysis of an aliquot of negolyte removed after aging revealed that the total concentration of DHAQ had declined by 16%. This decrease was mass-balanced by an increase in DHA DOI: 10.1021/jacs.8b13295 J. Am. Chem. Soc. 2019, 141, 8014−8019 2DHAHQ 4− + 2H2O → DHAQ 2− 3− − + DHA + 3OH Under the highly alkaline conditions within the negolyte compartment (Figure S11), facile one-electron oxidation of the DHA carbanion, followed by radical dimerization, would lead to (DHA)2. Indeed, pure samples of DHA are completely and irreversibly converted to (DHA)2 when subjected to electro- chemical oxidation under alkaline and anaerobic conditions (Figure S12). However, under aerobic conditions, oxidation of DHA back to DHAQ can be favored over dimerization (Figures 1A and S13), particularly at low DHA concentration (Figures S14 and S15). Precedents for the oxidation of anthrone species to anthraquinone and dianthrone species may be found in refs 45−56. The irreversible conversion of DHAHQ to (DHA)2 has a deleterious effect on battery operation due to the replacement of the anthraquinone moiety with a benzophenone functional group (Figures 1B and S16A). Although (DHA)2 is also redox- active, its use in a RFB is impractical for several reasons. First, the higher reduction potential of (DHA)2 with respect to 8015PDF Image | Extending organic flow batteries via redox state management
PDF Search Title:
Extending organic flow batteries via redox state managementOriginal File Name Searched:
mja287.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |