
PDF Publication Title:
Text from PDF Page: 025
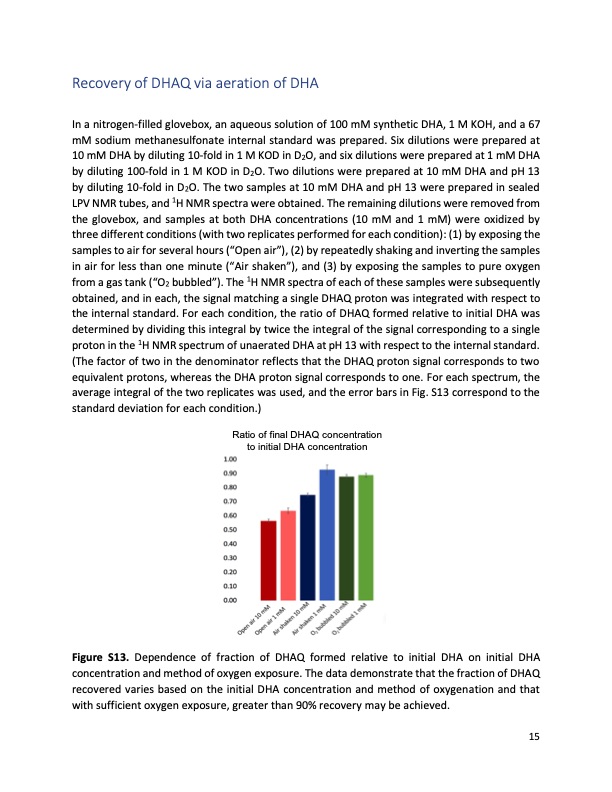
Recovery of DHAQ via aeration of DHA In a nitrogen-filled glovebox, an aqueous solution of 100 mM synthetic DHA, 1 M KOH, and a 67 mM sodium methanesulfonate internal standard was prepared. Six dilutions were prepared at 10 mM DHA by diluting 10-fold in 1 M KOD in D2O, and six dilutions were prepared at 1 mM DHA by diluting 100-fold in 1 M KOD in D2O. Two dilutions were prepared at 10 mM DHA and pH 13 by diluting 10-fold in D2O. The two samples at 10 mM DHA and pH 13 were prepared in sealed LPV NMR tubes, and 1H NMR spectra were obtained. The remaining dilutions were removed from the glovebox, and samples at both DHA concentrations (10 mM and 1 mM) were oxidized by three different conditions (with two replicates performed for each condition): (1) by exposing the samples to air for several hours (“Open air”), (2) by repeatedly shaking and inverting the samples in air for less than one minute (“Air shaken”), and (3) by exposing the samples to pure oxygen from a gas tank (“O2 bubbled”). The 1H NMR spectra of each of these samples were subsequently obtained, and in each, the signal matching a single DHAQ proton was integrated with respect to the internal standard. For each condition, the ratio of DHAQ formed relative to initial DHA was determined by dividing this integral by twice the integral of the signal corresponding to a single proton in the 1H NMR spectrum of unaerated DHA at pH 13 with respect to the internal standard. (The factor of two in the denominator reflects that the DHAQ proton signal corresponds to two equivalent protons, whereas the DHA proton signal corresponds to one. For each spectrum, the average integral of the two replicates was used, and the error bars in Fig. S13 correspond to the standard deviation for each condition.) Figure S13. Ratio of final DHAQ concentration to initial DHA concentration Dependence of fraction of DHAQ formed relative to initial DHA on initial DHA concentration and method of oxygen exposure. The data demonstrate that the fraction of DHAQ recovered varies based on the initial DHA concentration and method of oxygenation and that with sufficient oxygen exposure, greater than 90% recovery may be achieved. 15PDF Image | Extending organic flow batteries via redox state management
PDF Search Title:
Extending organic flow batteries via redox state managementOriginal File Name Searched:
mja287.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |