
PDF Publication Title:
Text from PDF Page: 028
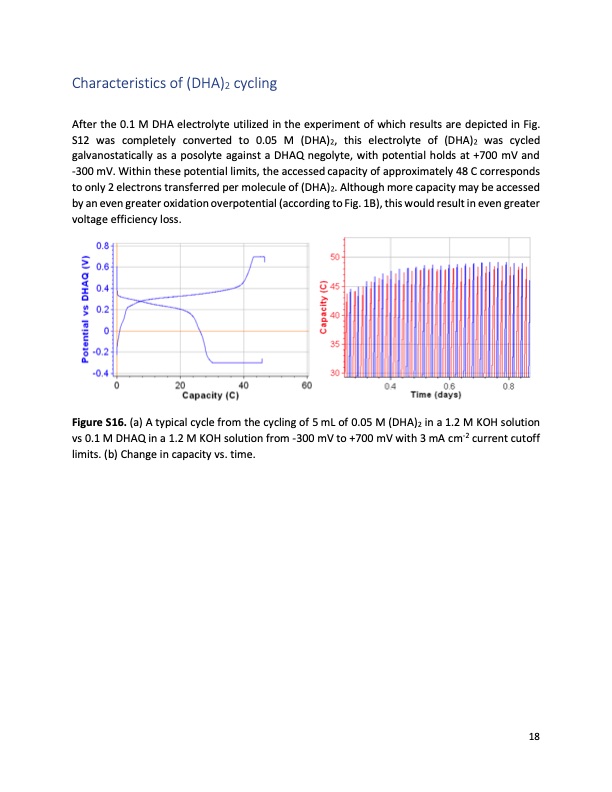
Characteristics of (DHA)2 cycling After the 0.1 M DHA electrolyte utilized in the experiment of which results are depicted in Fig. S12 was completely converted to 0.05 M (DHA)2, this electrolyte of (DHA)2 was cycled galvanostatically as a posolyte against a DHAQ negolyte, with potential holds at +700 mV and -300 mV. Within these potential limits, the accessed capacity of approximately 48 C corresponds to only 2 electrons transferred per molecule of (DHA)2. Although more capacity may be accessed by an even greater oxidation overpotential (according to Fig. 1B), this would result in even greater voltage efficiency loss. Figure S16. (a) A typical cycle from the cycling of vs 0.1 M DHAQ in a 1.2 M KOH solution from -300 mV to +700 mV with 3 mA cm-2 current cutoff 5 mL of 0.05 M (DHA)2 in a 1.2 M KOH solution limits. (b) Change in capacity vs. time. 18PDF Image | Extending organic flow batteries via redox state management
PDF Search Title:
Extending organic flow batteries via redox state managementOriginal File Name Searched:
mja287.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |