
PDF Publication Title:
Text from PDF Page: 032
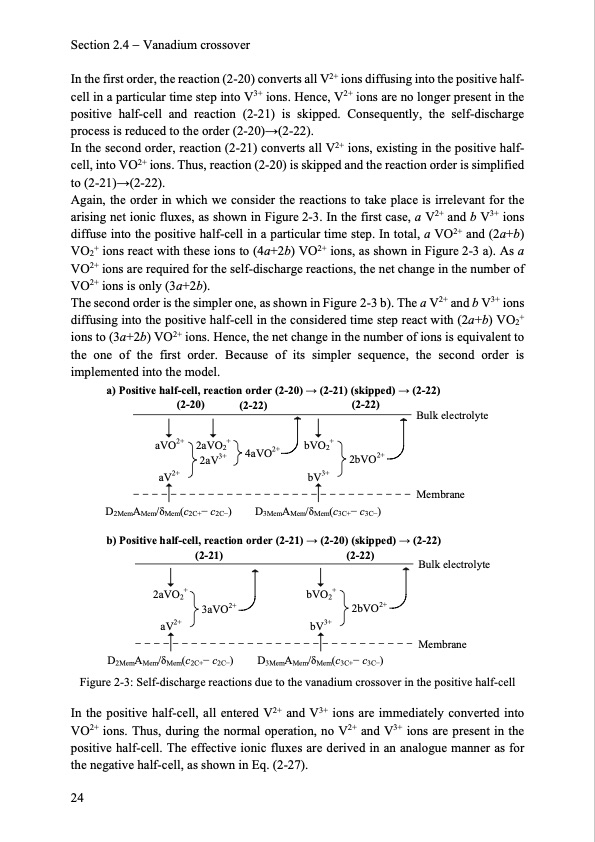
Section 2.4 Vanadium crossover In the first order, the reaction (2-20) converts all V2+ ions diffusing into the positive half- cell in a particular time step into V3+ ions. Hence, V2+ ions are no longer present in the positive half-cell and reaction (2-21) is skipped. Consequently, the self-discharge process is reduced to the order (2-20)→(2-22). In the second order, reaction (2-21) converts all V2+ ions, existing in the positive half- cell, into VO2+ ions. Thus, reaction (2-20) is skipped and the reaction order is simplified to (2-21)→(2-22). Again, the order in which we consider the reactions to take place is irrelevant for the arising net ionic fluxes, as shown in Figure 2-3. In the first case, a V2+ and b V3+ ions diffuse into the positive half-cell in a particular time step. In total, a VO2+ and (2a+b) VO2+ ions react with these ions to (4a+2b) VO2+ ions, as shown in Figure 2-3 a). As a VO2+ ions are required for the self-discharge reactions, the net change in the number of VO2+ ions is only (3a+2b). The second order is the simpler one, as shown in Figure 2-3 b). The a V2+ and b V3+ ions diffusing into the positive half-cell in the considered time step react with (2a+b) VO2+ ions to (3a+2b) VO2+ ions. Hence, the net change in the number of ions is equivalent to the one of the first order. Because of its simpler sequence, the second order is implemented into the model. a) Positive half-cell, reaction order (2-20) → (2-21) (skipped) → (2-22) (2-20) (2-22) aVO2+ 2aVO2+ 2+ 2aV3+ 4aVO aV2+ (2-22) bVO2+ 2bVO2+ bV3+ Bulk electrolyte Membrane D2MemAMem/δMem(c2C+− c2C−) b) Positive half-cell, reaction order (2-21) → (2-20) (skipped) → (2-22) (2-21) 2aVO2+ 3aVO2+ aV2+ D3MemAMem/δMem(c3C+− c3C−) (2-22) bVO2+ 2bVO2+ bV3+ Bulk electrolyte Membrane D2MemAMem/δMem(c2C+− c2C−) Figure 2-3: Self-discharge reactions due to the vanadium crossover in the positive half-cell In the positive half-cell, all entered V2+ and V3+ ions are immediately converted into VO2+ ions. Thus, during the normal operation, no V2+ and V3+ ions are present in the positive half-cell. The effective ionic fluxes are derived in an analogue manner as for the negative half-cell, as shown in Eq. (2-27). 24 D3MemAMem/δMem(c3C+− c3C−)PDF Image | Model-based Design Vanadium Redox Flow Batteries
PDF Search Title:
Model-based Design Vanadium Redox Flow BatteriesOriginal File Name Searched:
10-5445IR1000070670.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |