
PDF Publication Title:
Text from PDF Page: 119
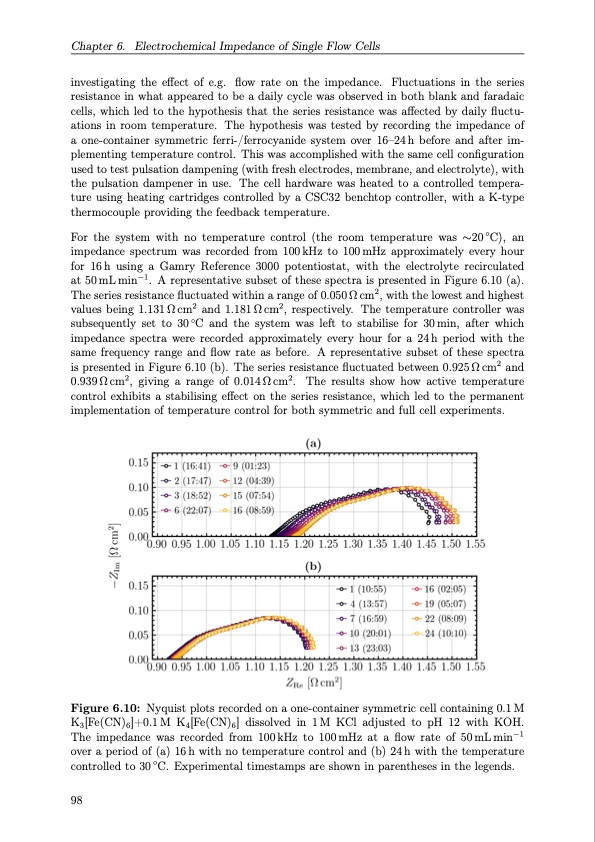
Chapter 6. Electrochemical Impedance of Single Flow Cells investigating the effect of e.g. flow rate on the impedance. Fluctuations in the series resistance in what appeared to be a daily cycle was observed in both blank and faradaic cells, which led to the hypothesis that the series resistance was affected by daily fluctu- ations in room temperature. The hypothesis was tested by recording the impedance of a one-container symmetric ferri-/ferrocyanide system over 16–24h before and after im- plementing temperature control. This was accomplished with the same cell configuration used to test pulsation dampening (with fresh electrodes, membrane, and electrolyte), with the pulsation dampener in use. The cell hardware was heated to a controlled tempera- ture using heating cartridges controlled by a CSC32 benchtop controller, with a K-type thermocouple providing the feedback temperature. For the system with no temperature control (the room temperature was ∼20◦C), an impedance spectrum was recorded from 100kHz to 100mHz approximately every hour for 16h using a Gamry Reference 3000 potentiostat, with the electrolyte recirculated at 50mLmin−1. A representative subset of these spectra is presented in Figure 6.10 (a). The series resistance fluctuated within a range of 0.050 Ω cm2, with the lowest and highest values being 1.131Ωcm2 and 1.181Ωcm2, respectively. The temperature controller was subsequently set to 30◦C and the system was left to stabilise for 30min, after which impedance spectra were recorded approximately every hour for a 24h period with the same frequency range and flow rate as before. A representative subset of these spectra is presented in Figure 6.10 (b). The series resistance fluctuated between 0.925 Ω cm2 and 0.939 Ω cm2, giving a range of 0.014 Ω cm2. The results show how active temperature control exhibits a stabilising effect on the series resistance, which led to the permanent implementation of temperature control for both symmetric and full cell experiments. Figure 6.10: Nyquist plots recorded on a one-container symmetric cell containing 0.1 M K3[Fe(CN)6]+0.1M K4[Fe(CN)6] dissolved in 1M KCl adjusted to pH 12 with KOH. The impedance was recorded from 100kHz to 100mHz at a flow rate of 50mLmin−1 over a period of (a) 16 h with no temperature control and (b) 24 h with the temperature controlled to 30 ◦C. Experimental timestamps are shown in parentheses in the legends. 98PDF Image | Organic Redox Flow Batteries 2023
PDF Search Title:
Organic Redox Flow Batteries 2023Original File Name Searched:
PhD_thesis_final_dorhoff_4_.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |