
PDF Publication Title:
Text from PDF Page: 015
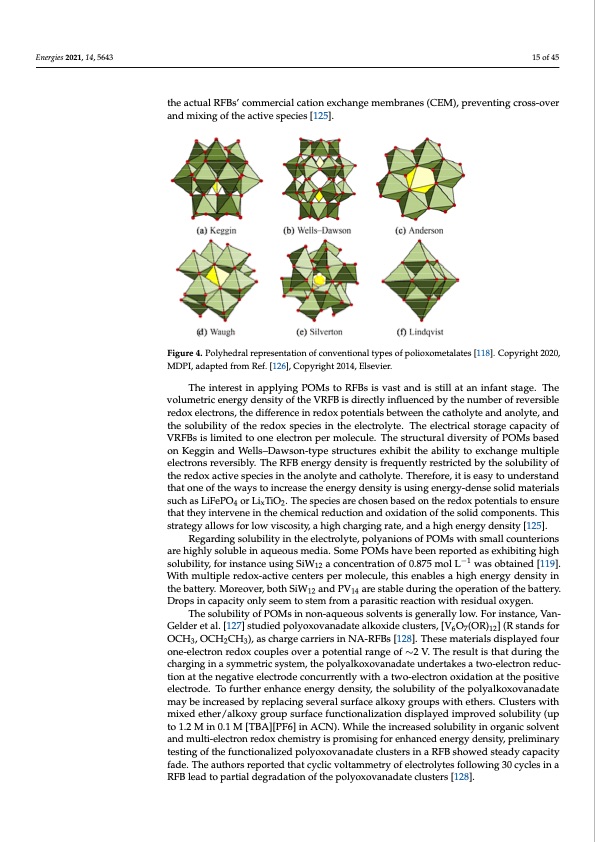
Energies 2021, 14, 5643 15 of 45 the actual RFBs’ commercial cation exchange membranes (CEM), preventing cross-over and mixing of the active species [125]. Figure 4. Polyhedral representation of conventional types of polioxometalates Figure 4. Polyhedral representation of conventional types of polioxometalates [118]. Copyright 2020, [119]. Copyright 2020, MDPI, adapted from Ref. [127], Copyright 2014, Elsevier. MDPI, adapted from Ref. [126], Copyright 2014, Elsevier. The interest in applying POMs to RFBs is vast and is still at an infant stage. The volumetric energy density of the VRFB is directly influenced by the number of reversible redox electrons, the difference in redox potentials between the catholyte and anolyte, and the solubility of the redox species in the electrolyte. The electrical storage capacity of VRFBs is limited to one electron per molecule. The structural diversity of POMs based on Keggin and Wells–Dawson-type structures exhibit the ability to exchange multiple electrons reversibly. The RFB energy density is frequently restricted by the solubility of the redox active species in the anolyte and catholyte. Therefore, it is easy to understand that one of the ways to increase the energy density is using energy-dense solid materials such as LiFePO4 or LixTiO2. The species are chosen based on the redox potentials to ensure that they intervene in the chemical reduction and oxidation of the solid components. This strategy allows for low viscosity, a high charging rate, and a high energy density [125]. Regarding solubility in the electrolyte, polyanions of POMs with small counterions are highly soluble in aqueous media. Some POMs have been reported as exhibiting high solubility, for instance using SiW12 a concentration of 0.875 mol L−1 was obtained [119]. With multiple redox-active centers per molecule, this enables a high energy density in the battery. Moreover, both SiW12 and PV14 are stable during the operation of the battery. Drops in capacity only seem to stem from a parasitic reaction with residual oxygen. The solubility of POMs in non-aqueous solvents is generally low. For instance, Van- Gelder et al. [127] studied polyoxovanadate alkoxide clusters, [V6O7(OR)12] (R stands for OCH3, OCH2CH3), as charge carriers in NA-RFBs [128]. These materials displayed four one-electron redox couples over a potential range of ∼2 V. The result is that during the charging in a symmetric system, the polyalkoxovanadate undertakes a two-electron reduc- tion at the negative electrode concurrently with a two-electron oxidation at the positive electrode. To further enhance energy density, the solubility of the polyalkoxovanadate [275]. (a) Schematic representation of the mentioned organic slurry RFB. (b) Illustration of the mixed ether/alkoxy group surface functionalization displayed improved solubility (up 1 Figure 11. Organic slurry RFB based on all polymer particulate suspension reported by Yan et al. may be increased by replacing several surface alkoxy groups with ethers. Clusters with proposed kinetic mechanism to elucidate the charge transfer particulates in the redox processes. (c) to 1.2 M in 0.1 M [TBA][PF6] in ACN). While the increased solubility in organic solvent The capacity, coulombic efficiency, and voltage efficiency in a galvanostatic charge–discharge cycle different flowrates. Copyright 2019, Springer Nature. and multi-electron redox chemistry is promising for enhanced energy density, preliminary for current densities from 5 mA cm−2 to 20 mA cm−2. (d) Representation of the long-term stability testing of the functionalized polyoxovanadate clusters in a RFB showed steady capacity during charge–discharge cycles at 20 mA cm−2. (e) Representation of the polarization curves for fade. The authors reported that cyclic voltammetry of electrolytes following 30 cycles in a RFB lead to partial degradation of the polyoxovanadate clusters [128].PDF Image | PNNL Vanadium Redox Flow Battery Stack
PDF Search Title:
PNNL Vanadium Redox Flow Battery StackOriginal File Name Searched:
energies-14-05643-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |