
PDF Publication Title:
Text from PDF Page: 029
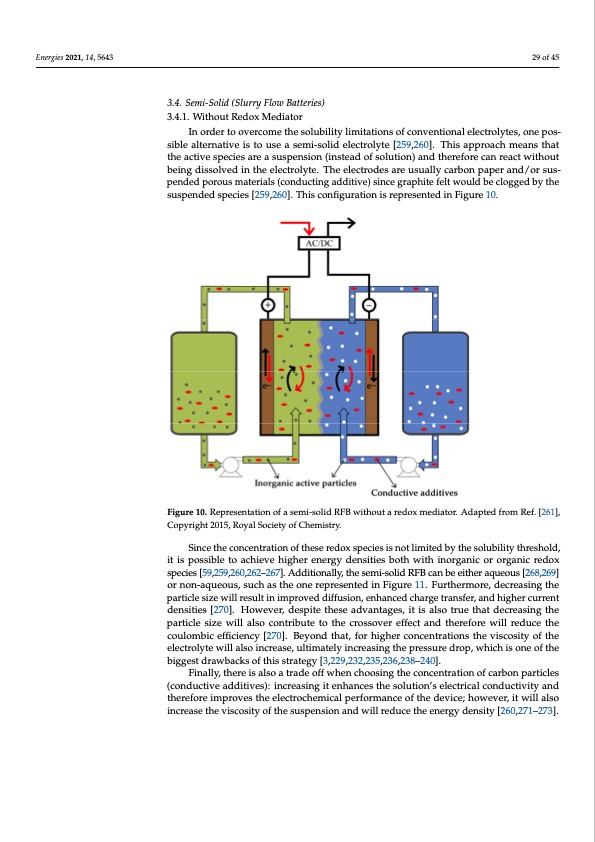
less, the extra care that must be taken with these types of batteries to ensure safe use and Energies 2021, 14, 5643 long life increase their price, making them lose the main advantages against batteries that use more expensive materials. Even though these batteries are commercially available, they do not seem a promising a long-term solution, and will be replaced as soon as an improved low-cost option becomes available. 29 of 45 3.4. Semi-Solid (Slurry Flow Batteries) 3.4.1. Without Redox Mediator 3.4. Semi-Solid (Slurry Flow Batteries) In order to overcome the solubility limitations of conventional electrolytes, one pos- 3.4.1. Without Redox Mediator sible alternative is to use a semi-solid electrolyte [260,261]. This approach means that the In order to overcome the solubility limitations of conventional electrolytes, one pos- active species are a suspension (instead of solution) and therefore can react without being sible alternative is to use a semi-solid electrolyte [259,260]. This approach means that dissolved in the electrolyte. The electrodes are usually carbon paper and/or suspended the active species are a suspension (instead of solution) and therefore can react without porous materials (conducting additive) since graphite felt would be clogged by the sus- being dissolved in the electrolyte. The electrodes are usually carbon paper and/or sus- pended species [260,261]. This configuration is represented in Figure 10. pended porous materials (conducting additive) since graphite felt would be clogged by the suspended species [259,260]. This configuration is represented in Figure 10. Figure 10. Representation of a semi-solid RFB without a redox mediator. Adapted from Ref. [262], Figure 10. Representation of a semi-solid RFB without a redox mediator. Adapted from Ref. [261], Copyright 2015, Royal Society of Chemistry. Copyright 2015, Royal Society of Chemistry. Since the concentration of these redox species is not limited by the solubility threshold, it is possible to achieve higher energy densities both with inorganic or organic redox species [59,259,260,262–267]. Additionally, the semi-solid RFB can be either aqueous [268,269] or non-aqueous, such as the one represented in Figure 11. Furthermore, decreasing the particle size will result in improved diffusion, enhanced charge transfer, and higher current densities [270]. However, despite these advantages, it is also true that decreasing the particle size will also contribute to the crossover effect and therefore will reduce the coulombic efficiency [270]. Beyond that, for higher concentrations the viscosity of the electrolyte will also increase, ultimately increasing the pressure drop, which is one of the biggest drawbacks of this strategy [3,229,232,235,236,238–240]. Finally, there is also a trade off when choosing the concentration of carbon particles (conductive additives): increasing it enhances the solution’s electrical conductivity and therefore improves the electrochemical performance of the device; however, it will also increase the viscosity of the suspension and will reduce the energy density [260,271–273].PDF Image | PNNL Vanadium Redox Flow Battery Stack
PDF Search Title:
PNNL Vanadium Redox Flow Battery StackOriginal File Name Searched:
energies-14-05643-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |