
PDF Publication Title:
Text from PDF Page: 031
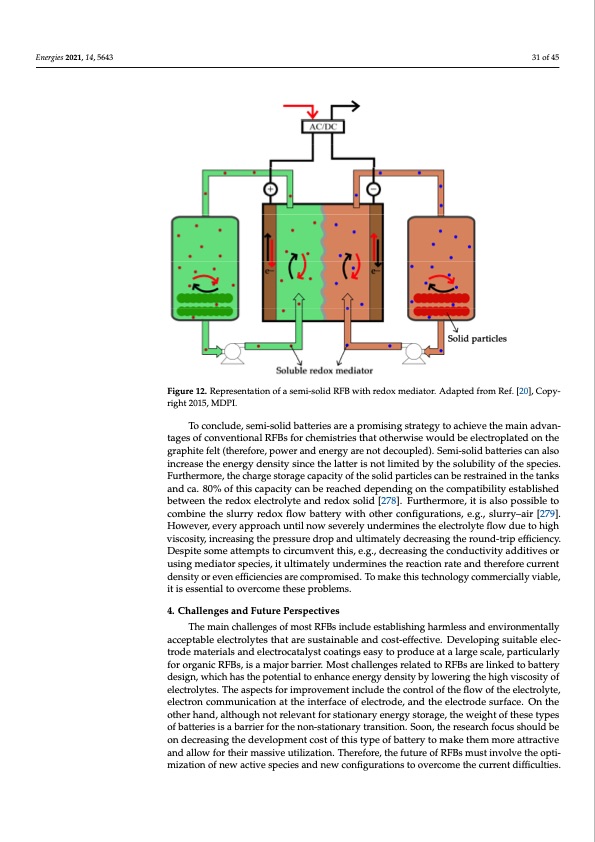
Energies 2021, 14, 5643 means that the main redox species do not flow with the electrolyte, being restricted on the reservoirs. Instead, a redox mediator (secondary redox species) flows into the cell, reacts when in contact with the bipolar plate, and then goes back to the tank and reacts with the main redox species [5,267,276]. This principle is schematized in Figure 12. 31 of 45 Figure 12. Representation of a semi-solid RFB with redox mediator. Adapted from Ref. [20], Copy- Figure 12. Representation of a semi-solid RFB with redox mediator. Adapted from Ref. [20], Copy- right 2015, MDPI. right 2015, MDPI. Therefore, it is possible to store energy on the main species while relying on the flu- To conclude, semi-solid batteries are a promising strategy to achieve the main advan- idityagaensdofvcisocnovseintytionfathleRFreBdsofoxrmcheedmiaitsotr,iensotthoatnolythdeerwcriesaeswinogutlhdebperelsescutroepdlartoepdbountthaleso enhgaranpchinitge ftehlet (cthearegfeorter,apnoswpeortanadndentehrguys athrenpoot wdecrouopultepdu).t S[e2m67i-,2so7l2id]. bTahttermieasjcoarndarlasow- baciknscroefastheitshaepepnreoragcyhdiesntshiatytsincetherleatatreertiwsnooktilnimetitcedprboycethsseesoilnuvboilivtyedof(itnheceslpleacnidesi.n tanks), the device electrochemical performance tends to decrease (coulombic and voltage Furthermore, the charge storage capacity of the solid particles can be restrained in the tanks efficiency, current/power density), despite there still being some divergence about this and ca. 80% of this capacity can be reached depending on the compatibility established between the redox electrolyte and redox solid [278]. Furthermore, it is also possible to topic [53,277]. Additionally, this double kinetics system leads to a dependence between combine the slurry redox flow battery with other configurations, e.g., slurry–air [279]. power and capacity [272,278] and the screening process of choosing mediator and active However, every approach until now severely undermines the electrolyte flow due to high species gets even harder when compared to other electrolytes [272]. viscosity, increasing the pressure drop and ultimately decreasing the round-trip efficiency. To conclude, semi-solid batteries are a promising strategy to achieve the main ad- Despite some attempts to circumvent this, e.g., decreasing the conductivity additives or vantages of conventional RFBs for chemistries that otherwise would be electroplated on using mediator species, it ultimately undermines the reaction rate and therefore current the graphite felt (therefore, power and energy are not decoupled). Semi-solid batteries can density or even efficiencies are compromised. To make this technology commercially viable, also increase the energy density since the latter is not limited by the solubility of the spe- it is essential to overcome these problems. cies. Furthermore, the charge storage capacity of the solid particles can be restrained in the4t.aCnhkasllaendgecsa.a8n0d%FuotfutrheisPecraspaecitiyvecsan be reached depending on the compatibility es- tablished between the redox electrolyte and redox solid [279]. Furthermore, it is also pos- The main challenges of most RFBs include establishing harmless and environmentally sibaleccteopctaobmlebienlectrhoelystleusrrtyharteadroexsuflsotwainbaabtlterayndwcitohsto-etfhferctcivoen.fiDgeuvraeltoiopnins,ges.ugi.,tasblulerreyle–ca-ir [28t0r]o.dHeomwaetevreira,lsevanerdyealepcptrocaacthaluysntticlonaotiwngseevaesryeltyo purnodeurcmeianteas ltahregelseccatlreo,lpyatertficlouwlardlyue to high viscosity, increasing the pressure drop and ultimately decreasing the round-trip for organic RFBs, is a major barrier. Most challenges related to RFBs are linked to battery efficiency. Despite some attempts to circumvent this, e.g., decreasing the conductivity ad- design, which has the potential to enhance energy density by lowering the high viscosity of ditives or using mediator species, it ultimately undermines the reaction rate and therefore electrolytes. The aspects for improvement include the control of the flow of the electrolyte, electron communication at the interface of electrode, and the electrode surface. On the current density or even efficiencies are compromised. To make this technology commer- other hand, although not relevant for stationary energy storage, the weight of these types cially viable, it is essential to overcome these problems. of batteries is a barrier for the non-stationary transition. Soon, the research focus should be on decreasing the development cost of this type of battery to make them more attractive and allow for their massive utilization. Therefore, the future of RFBs must involve the opti- mization of new active species and new configurations to overcome the current difficulties.PDF Image | PNNL Vanadium Redox Flow Battery Stack
PDF Search Title:
PNNL Vanadium Redox Flow Battery StackOriginal File Name Searched:
energies-14-05643-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |