
PDF Publication Title:
Text from PDF Page: 007
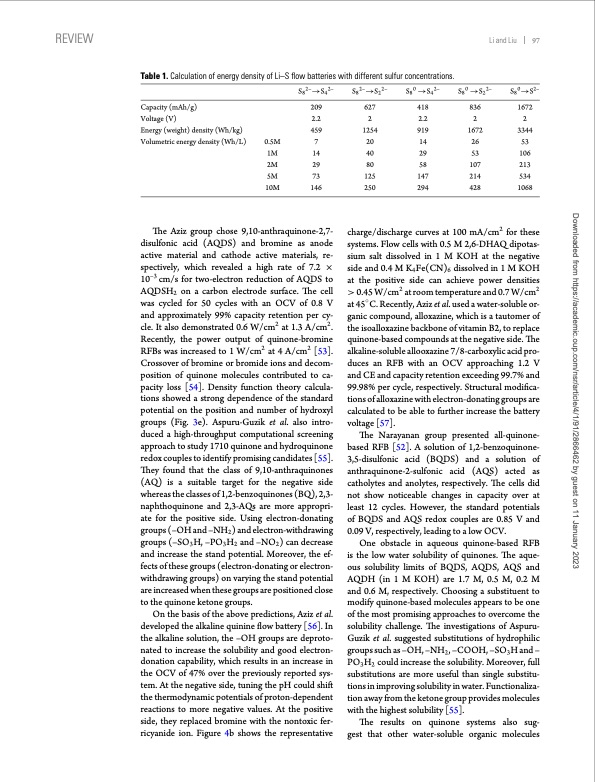
REVIEW Li and Liu 97 Table 1. Calculation of energy density of Li–S flow batteries with different sulfur concentrations. S82–→S42– S82–→S22– S80 →S42– Capacity (mAh/g) Voltage (V) Energy (weight) density (Wh/kg) Volumetric energy density (Wh/L) S80 →S22– S80→S2– 209 627 418 2.2 2 2.2 2 2 459 1254 919 1672 3344 7 20 14 26 53 1M 14 40 29 53 106 0.5M 2M 29 80 58 107 213 5M 73 10M 146 125 147 214 534 250 294 428 1068 charge/discharge curves at 100 mA/cm2 for these systems. Flow cells with 0.5 M 2,6-DHAQ dipotas- sium salt dissolved in 1 M KOH at the negative side and 0.4 M K4Fe(CN)6 dissolved in 1 M KOH at the positive side can achieve power densities > 0.45 W/cm2 at room temperature and 0.7 W/cm2 at 45◦ C. Recently, Aziz et al. used a water-soluble or- ganic compound, alloxazine, which is a tautomer of the isoalloxazine backbone of vitamin B2, to replace quinone-based compounds at the negative side. The alkaline-soluble allooxazine 7/8-carboxylic acid pro- duces an RFB with an OCV approaching 1.2 V and CE and capacity retention exceeding 99.7% and 99.98% per cycle, respectively. Structural modifica- tions of alloxazine with electron-donating groups are calculated to be able to further increase the battery voltage [57]. The Narayanan group presented all-quinone- based RFB [52]. A solution of 1,2-benzoquinone- 3,5-disulfonic acid (BQDS) and a solution of anthraquinone-2-sulfonic acid (AQS) acted as catholytes and anolytes, respectively. The cells did not show noticeable changes in capacity over at least 12 cycles. However, the standard potentials of BQDS and AQS redox couples are 0.85 V and 0.09 V, respectively, leading to a low OCV. One obstacle in aqueous quinone-based RFB is the low water solubility of quinones. The aque- ous solubility limits of BQDS, AQDS, AQS and AQDH(in1MKOH)are1.7M,0.5M,0.2M and 0.6 M, respectively. Choosing a substituent to modify quinone-based molecules appears to be one of the most promising approaches to overcome the solubility challenge. The investigations of Aspuru- Guzik et al. suggested substitutions of hydrophilic groups such as –OH, –NH2 , –COOH, –SO3 H and – PO3H2 could increase the solubility. Moreover, full substitutions are more useful than single substitu- tions in improving solubility in water. Functionaliza- tion away from the ketone group provides molecules with the highest solubility [55]. The results on quinone systems also sug- gest that other water-soluble organic molecules 836 1672 The Aziz group chose 9,10-anthraquinone-2,7- disulfonic acid (AQDS) and bromine as anode active material and cathode active materials, re- spectively, which revealed a high rate of 7.2 × 10–3 cm/s for two-electron reduction of AQDS to AQDSH2 on a carbon electrode surface. The cell was cycled for 50 cycles with an OCV of 0.8 V and approximately 99% capacity retention per cy- cle. It also demonstrated 0.6 W/cm2 at 1.3 A/cm2. Recently, the power output of quinone-bromine RFBs was increased to 1 W/cm2 at 4 A/cm2 [53]. Crossover of bromine or bromide ions and decom- position of quinone molecules contributed to ca- pacity loss [54]. Density function theory calcula- tions showed a strong dependence of the standard potential on the position and number of hydroxyl groups (Fig. 3e). Aspuru-Guzik et al. also intro- duced a high-throughput computational screening approach to study 1710 quinone and hydroquinone redox couples to identify promising candidates [55]. They found that the class of 9,10-anthraquinones (AQ) is a suitable target for the negative side whereas the classes of 1,2-benzoquinones (BQ), 2,3- naphthoquinone and 2,3-AQs are more appropri- ate for the positive side. Using electron-donating groups (–OH and –NH2 ) and electron-withdrawing groups (–SO3 H, –PO3 H2 and –NO2 ) can decrease and increase the stand potential. Moreover, the ef- fects of these groups (electron-donating or electron- withdrawing groups) on varying the stand potential are increased when these groups are positioned close to the quinone ketone groups. On the basis of the above predictions, Aziz et al. developed the alkaline quinine flow battery [56]. In the alkaline solution, the –OH groups are deproto- nated to increase the solubility and good electron- donation capability, which results in an increase in the OCV of 47% over the previously reported sys- tem. At the negative side, tuning the pH could shift the thermodynamic potentials of proton-dependent reactions to more negative values. At the positive side, they replaced bromine with the nontoxic fer- ricyanide ion. Figure 4b shows the representative Downloaded from https://academic.oup.com/nsr/article/4/1/91/2866462 by guest on 11 January 2023PDF Image | Progress in low cost redox flow batteries energy storage
PDF Search Title:
Progress in low cost redox flow batteries energy storageOriginal File Name Searched:
nww098.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |