
PDF Publication Title:
Text from PDF Page: 009
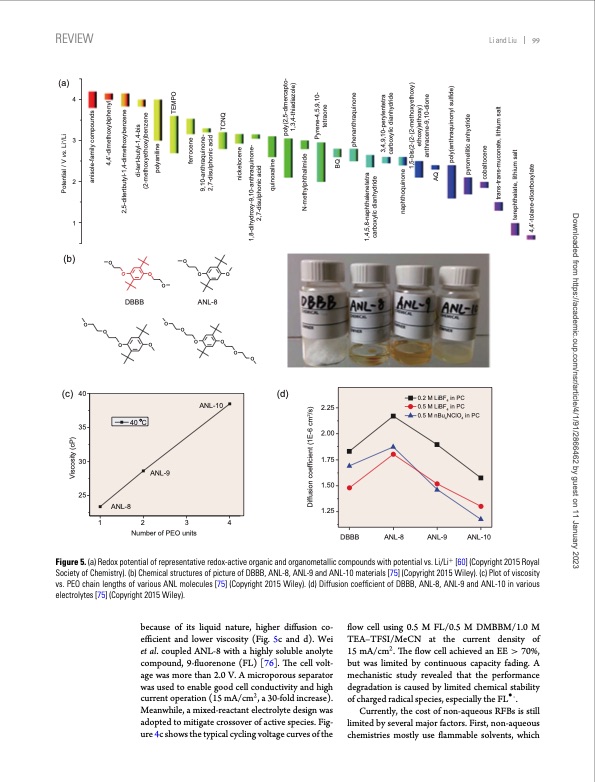
REVIEW Li and Liu 99 (a) 4 3 2 1 (b)o o ooo o (c) 40 35 30 25 (d) DBBB ANL-8 oo o o oo oo o o 0.2 M LiBF in PC 4 0.5 M LiBF in PC 4 0.5 M nBu NClO in PC 44 ANL-8 40 C ANL-9 ANL-10 1234 Number of PEO units DBBB ANL-8 ANL-9 ANL-10 Figure 5. (a) Redox potential of representative redox-active organic and organometallic compounds with potential vs. Li/Li+ [60] (Copyright 2015 Royal Society of Chemistry). (b) Chemical structures of picture of DBBB, ANL-8, ANL-9 and ANL-10 materials [75] (Copyright 2015 Wiley). (c) Plot of viscosity vs. PEO chain lengths of various ANL molecules [75] (Copyright 2015 Wiley). (d) Diffusion coefficient of DBBB, ANL-8, ANL-9 and ANL-10 in various electrolytes [75] (Copyright 2015 Wiley). because of its liquid nature, higher diffusion co- efficient and lower viscosity (Fig. 5c and d). Wei et al. coupled ANL-8 with a highly soluble anolyte compound, 9-fluorenone (FL) [76]. The cell volt- age was more than 2.0 V. A microporous separator was used to enable good cell conductivity and high current operation (15 mA/cm2, a 30-fold increase). Meanwhile, a mixed-reactant electrolyte design was adopted to mitigate crossover of active species. Fig- ure 4c shows the typical cycling voltage curves of the flow cell using 0.5 M FL/0.5 M DMBBM/1.0 M TEA–TFSI/MeCN at the current density of 15 mA/cm2. The flow cell achieved an EE > 70%, but was limited by continuous capacity fading. A mechanistic study revealed that the performance degradation is caused by limited chemical stability of charged radical species, especially the FL r-. Currently, the cost of non-aqueous RFBs is still limited by several major factors. First, non-aqueous chemistries mostly use flammable solvents, which 2.25 2.00 1.75 1.50 1.25 o Viscosity (cP) Diffusion coefficient (1E-6 cm2/s) N-methylphthalimide 1,4,5,8-naphthalenetetra carboxylic dianhydride Potential / V vs. Li+/Li 1,8-dihydroxy-9,10-anthraquinone- 2,7-disulphonic acid anisole-family compounds 4,4’-dimethoxybiphenyl 2,5-ditertbutyl-1,4-dimethoxybenzene di-tert-butyl-1,4-bis (2-methoxyethoxy)benzene quinoxaline polyaniline ferrocene 9,10-anthraquinone- 2,7-disulphonic acid nickelocene TCNQ TEMPO poly(2,5-dimercapto- 1,3,4-thiadiazole) Pyrene-4,5,9,10- tetraone BQ phenanthraquinone 3,4,9,10-perylentetra carboxylic dianhydride naphthoquinone 1,5-bis(2-(2-(2-methoxyethoxy) ethoxy)ethoxy) anthracene-9,10-dione AQ poly(anthraquinonyl sulfide) pyromellitic anhydride cobaltocene trans-trans-muconate, lithium salt terephthalate, lithium salt 4,4’-tolane-dicarboxylate Downloaded from https://academic.oup.com/nsr/article/4/1/91/2866462 by guest on 11 January 2023PDF Image | Progress in low cost redox flow batteries energy storage
PDF Search Title:
Progress in low cost redox flow batteries energy storageOriginal File Name Searched:
nww098.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |