
PDF Publication Title:
Text from PDF Page: 020
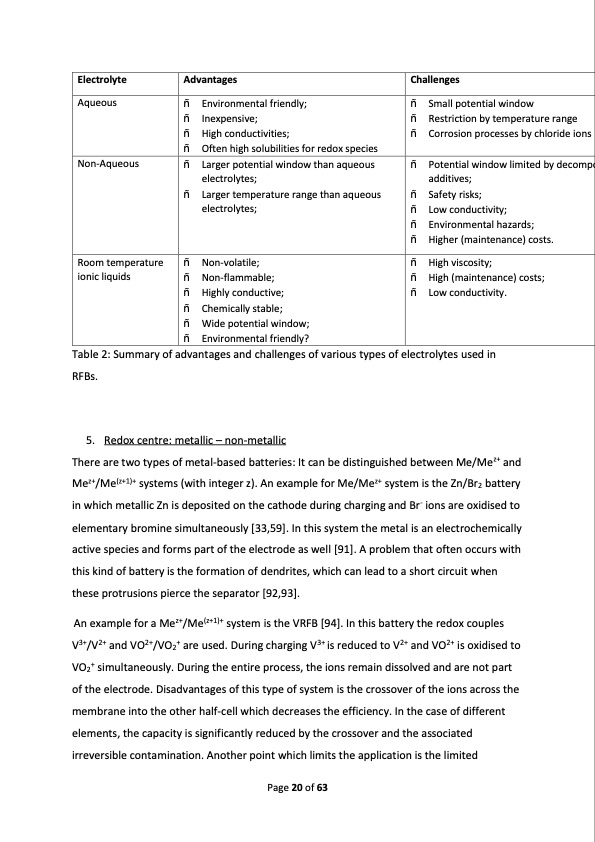
Electrolyte Advantages Challenges � Environmental friendly; � Inexpensive; � High conductivities; � Often high solubilities for redox species � Small potential window � Restriction by temperature range � Corrosion processes by chloride ions Aqueous � Larger potential window than aqueous electrolytes; � Larger temperature range than aqueous electrolytes; Table 2: Summary of advantages and challenges of various types of electrolytes used in RFBs. 5. Redox centre: metallic � non-metallic There are two types of metal-based batteries: It can be distinguished between Me/Mez+ and Mez+/Me(z+1)+ systems (with integer z). An example for Me/Mez+ system is the Zn/Br2 battery in which metallic Zn is deposited on the cathode during charging and Br- ions are oxidised to elementary bromine simultaneously [33,59]. In this system the metal is an electrochemically active species and forms part of the electrode as well [91]. A problem that often occurs with this kind of battery is the formation of dendrites, which can lead to a short circuit when these protrusions pierce the separator [92,93]. An example for a Mez+/Me(z+1)+ system is the VRFB [94]. In this battery the redox couples V3+/V2+ and VO2+/VO2+ are used. During charging V3+ is reduced to V2+ and VO2+ is oxidised to VO2+ simultaneously. During the entire process, the ions remain dissolved and are not part of the electrode. Disadvantages of this type of system is the crossover of the ions across the membrane into the other half-cell which decreases the efficiency. In the case of different elements, the capacity is significantly reduced by the crossover and the associated irreversible contamination. Another point which limits the application is the limited � Potential window limited by decomp additives; � Safety risks; � Low conductivity; � Environmental hazards; � Higher (maintenance) costs. Non-Aqueous � Non-volatile; � Non-flammable; � Highly conductive; � Chemically stable; � Wide potential window; � Environmental friendly? � High viscosity; � High (maintenance) costs; � Low conductivity. Room temperature ionic liquids Page 20 of 63 oPDF Image | Redox Flow Batteries Concepts Chemistries
PDF Search Title:
Redox Flow Batteries Concepts ChemistriesOriginal File Name Searched:
5870EAF5-2D70-44C8-A0A7-62D3A1462269.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |