
PDF Publication Title:
Text from PDF Page: 014
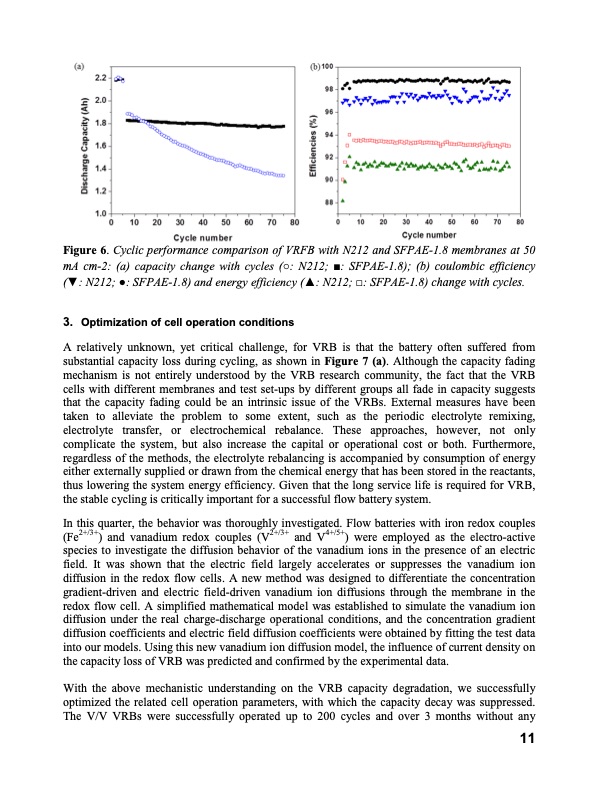
Figure 6. Cyclic performance comparison of VRFB with N212 and SFPAE-1.8 membranes at 50 mA cm-2: (a) capacity change with cycles (○: N212; ■: SFPAE-1.8); (b) coulombic efficiency (▼: N212; ●: SFPAE-1.8) and energy efficiency (▲: N212; □: SFPAE-1.8) change with cycles. 3. Optimizationofcelloperationconditions A relatively unknown, yet critical challenge, for VRB is that the battery often suffered from substantial capacity loss during cycling, as shown in Figure 7 (a). Although the capacity fading mechanism is not entirely understood by the VRB research community, the fact that the VRB cells with different membranes and test set-ups by different groups all fade in capacity suggests that the capacity fading could be an intrinsic issue of the VRBs. External measures have been taken to alleviate the problem to some extent, such as the periodic electrolyte remixing, electrolyte transfer, or electrochemical rebalance. These approaches, however, not only complicate the system, but also increase the capital or operational cost or both. Furthermore, regardless of the methods, the electrolyte rebalancing is accompanied by consumption of energy either externally supplied or drawn from the chemical energy that has been stored in the reactants, thus lowering the system energy efficiency. Given that the long service life is required for VRB, the stable cycling is critically important for a successful flow battery system. In this quarter, the behavior was thoroughly investigated. Flow batteries with iron redox couples (Fe2+/3+) and vanadium redox couples (V2+/3+ and V4+/5+) were employed as the electro-active species to investigate the diffusion behavior of the vanadium ions in the presence of an electric field. It was shown that the electric field largely accelerates or suppresses the vanadium ion diffusion in the redox flow cells. A new method was designed to differentiate the concentration gradient-driven and electric field-driven vanadium ion diffusions through the membrane in the redox flow cell. A simplified mathematical model was established to simulate the vanadium ion diffusion under the real charge-discharge operational conditions, and the concentration gradient diffusion coefficients and electric field diffusion coefficients were obtained by fitting the test data into our models. Using this new vanadium ion diffusion model, the influence of current density on the capacity loss of VRB was predicted and confirmed by the experimental data. With the above mechanistic understanding on the VRB capacity degradation, we successfully optimized the related cell operation parameters, with which the capacity decay was suppressed. The V/V VRBs were successfully operated up to 200 cycles and over 3 months without any 11PDF Image | Redox Flow Batteries for Stationary Electrical Energy Storage
PDF Search Title:
Redox Flow Batteries for Stationary Electrical Energy StorageOriginal File Name Searched:
PNNL-21174.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |