
PDF Publication Title:
Text from PDF Page: 084
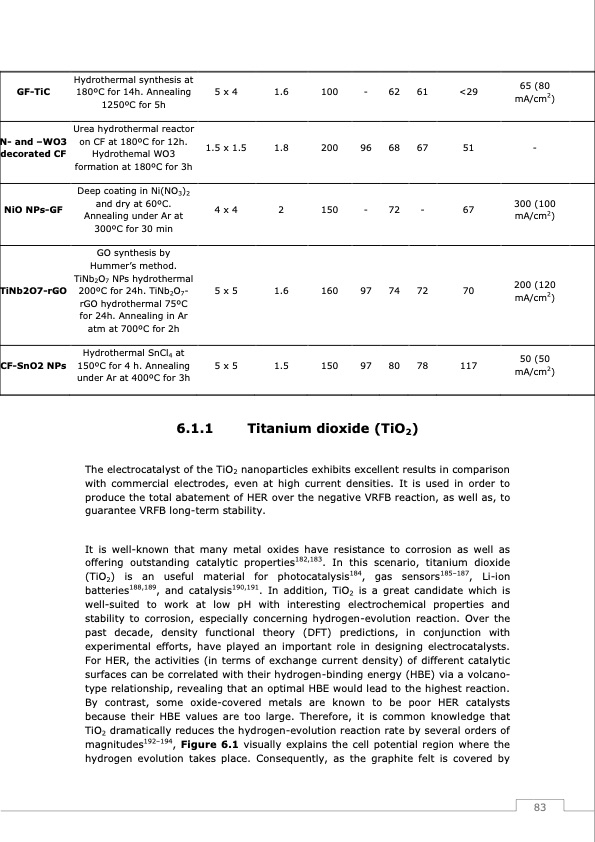
GF-TiC N- and –WO3 decorated CF NiO NPs-GF TiNb2O7-rGO CF-SnO2 NPs Hydrothermal synthesis at 180oC for 14h. Annealing 1250oC for 5h Urea hydrothermal reactor on CF at 180oC for 12h. Hydrothemal WO3 formation at 180oC for 3h Deep coating in Ni(NO3)2 anddryat60oC. Annealing under Ar at 300oC for 30 min GO synthesis by Hummer’s method. TiNb2O7 NPs hydrothermal 200oC for 24h. TiNb2O7- rGO hydrothermal 75oC for 24h. Annealing in Ar atm at 700oC for 2h Hydrothermal SnCl4 at 150oC for 4 h. Annealing under Ar at 400oC for 3h 5 x 4 1.5 x 1.5 4x4 5 x 5 5 x 5 1.6 100 1.8 200 2 150 1.6 160 1.5 150 - 62 96 68 61 <29 65 (80 mA/cm2) 67 51 - - 72 - 67 97 74 72 70 97 80 78 117 300 (100 mA/cm2) 200 (120 mA/cm2) 50 (50 mA/cm2) 6.1.1 Titanium dioxide (TiO2) The electrocatalyst of the TiO2 nanoparticles exhibits excellent results in comparison with commercial electrodes, even at high current densities. It is used in order to produce the total abatement of HER over the negative VRFB reaction, as well as, to guarantee VRFB long-term stability. It is well-known that many metal oxides have resistance to corrosion as well as offering outstanding catalytic properties182,183. In this scenario, titanium dioxide (TiO2) is an useful material for photocatalysis184, gas sensors185–187, Li-ion batteries188,189, and catalysis190,191. In addition, TiO2 is a great candidate which is well-suited to work at low pH with interesting electrochemical properties and stability to corrosion, especially concerning hydrogen-evolution reaction. Over the past decade, density functional theory (DFT) predictions, in conjunction with experimental efforts, have played an important role in designing electrocatalysts. For HER, the activities (in terms of exchange current density) of different catalytic surfaces can be correlated with their hydrogen-binding energy (HBE) via a volcano- type relationship, revealing that an optimal HBE would lead to the highest reaction. By contrast, some oxide-covered metals are known to be poor HER catalysts because their HBE values are too large. Therefore, it is common knowledge that TiO2 dramatically reduces the hydrogen-evolution reaction rate by several orders of magnitudes192–194, Figure 6.1 visually explains the cell potential region where the hydrogen evolution takes place. Consequently, as the graphite felt is covered by 83PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |