
PDF Publication Title:
Text from PDF Page: 119
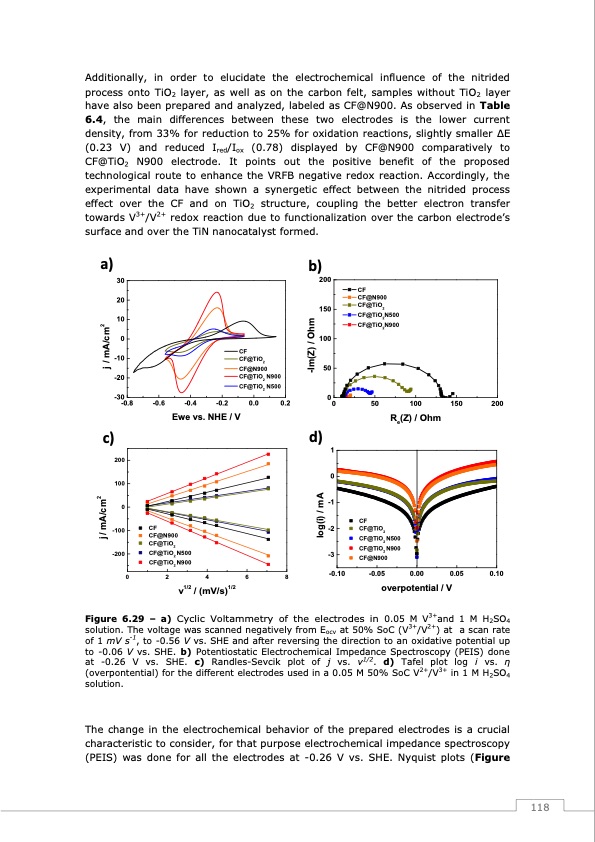
Additionally, in order to elucidate the electrochemical influence of the nitrided process onto TiO2 layer, as well as on the carbon felt, samples without TiO2 layer have also been prepared and analyzed, labeled as CF@N900. As observed in Table 6.4, the main differences between these two electrodes is the lower current density, from 33% for reduction to 25% for oxidation reactions, slightly smaller ΔE (0.23 V) and reduced Ired/Iox (0.78) displayed by CF@N900 comparatively to CF@TiO2 N900 electrode. It points out the positive benefit of the proposed technological route to enhance the VRFB negative redox reaction. Accordingly, the experimental data have shown a synergetic effect between the nitrided process effect over the CF and on TiO2 structure, coupling the better electron transfer towards V3+/V2+ redox reaction due to functionalization over the carbon electrode’s surface and over the TiN nanocatalyst formed. a) b) 200 150 100 50 0 d) 1 0 -1 c) 200 100 0 -100 -200 CF -2 CF@N900 CF@TiO2 CF@TiO N500 -3 2 CF CF@TiO2 30 20 10 0 -10 -20 -30 -0.8 CF CF@N900 CF@TiO2 CF@TiO2N500 CF@TiO2N900 -0.6 -0.4 -0.2 0.0 0.2 0 50 100 150 200 Re(Z) / Ohm Ewe vs. NHE / V CF CF@TiO2 CF@N900 CF@TiO2 N900 CF@TiO2 N500 j / mA/cm2 j / mA/cm2 log(i) / mA -Im(Z) / Ohm CF@TiO2 N900 02468 v1/2 / (mV/s)1/2 -0.10 CF@TiO2 N500 CF@TiO2 N900 CF@N900 -0.05 0.00 0.05 0.10 overpotential / V Figure 6.29 – a) Cyclic Voltammetry of the electrodes in 0.05 M V3+and 1 M H2SO4 solution. The voltage was scanned negatively from Eocv at 50% SoC (V3+/V2+) at a scan rate of 1 mV s-1, to -0.56 V vs. SHE and after reversing the direction to an oxidative potential up to -0.06 V vs. SHE. b) Potentiostatic Electrochemical Impedance Spectroscopy (PEIS) done at -0.26 V vs. SHE. c) Randles-Sevcik plot of j vs. ν1/2. d) Tafel plot log i vs. η (overpontential) for the different electrodes used in a 0.05 M 50% SoC V2+/V3+ in 1 M H2SO4 solution. The change in the electrochemical behavior of the prepared electrodes is a crucial characteristic to consider, for that purpose electrochemical impedance spectroscopy (PEIS) was done for all the electrodes at -0.26 V vs. SHE. Nyquist plots (Figure 118PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |