
PDF Publication Title:
Text from PDF Page: 130
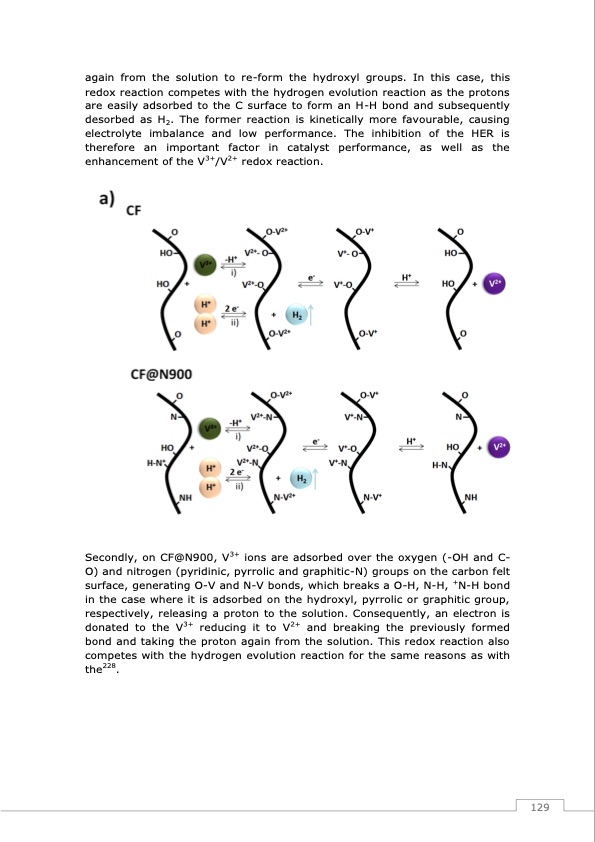
again from the solution to re-form the hydroxyl groups. In this case, this redox reaction competes with the hydrogen evolution reaction as the protons are easily adsorbed to the C surface to form an H-H bond and subsequently desorbed as H2. The former reaction is kinetically more favourable, causing electrolyte imbalance and low performance. The inhibition of the HER is therefore an important factor in catalyst performance, as well as the enhancement of the V3+/V2+ redox reaction. Secondly, on CF@N900, V3+ ions are adsorbed over the oxygen (-OH and C- O) and nitrogen (pyridinic, pyrrolic and graphitic-N) groups on the carbon felt surface, generating O-V and N-V bonds, which breaks a O-H, N-H, +N-H bond in the case where it is adsorbed on the hydroxyl, pyrrolic or graphitic group, respectively, releasing a proton to the solution. Consequently, an electron is donated to the V3+ reducing it to V2+ and breaking the previously formed bond and taking the proton again from the solution. This redox reaction also competes with the hydrogen evolution reaction for the same reasons as with the228. 129PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |