
PDF Publication Title:
Text from PDF Page: 133
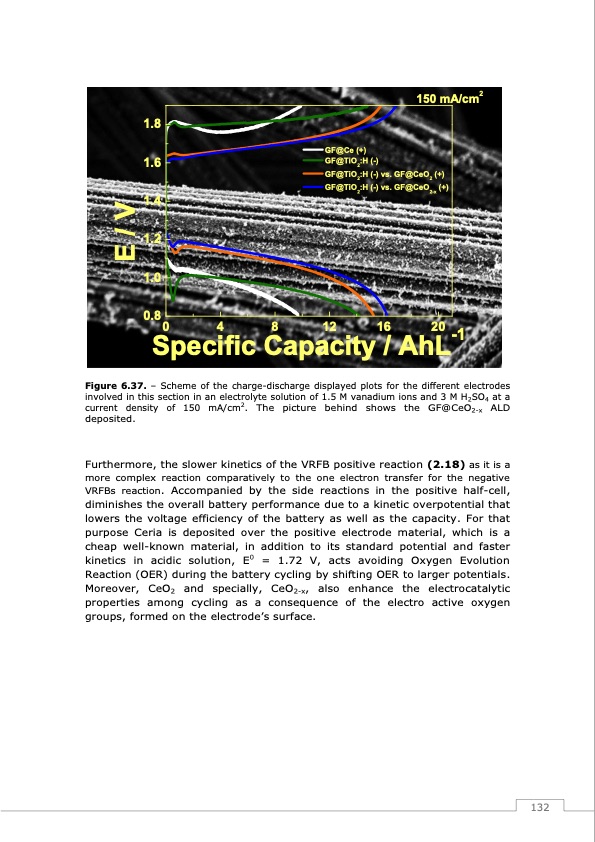
1.8 1.6 1.4 1.2 1.0 GF@Ce (+) GF@TiO2:H (-) 0.80 4 8 12 16 20-1 Specific Capacity / AhL Figure 6.37. – Scheme of the charge-discharge displayed plots for the different electrodes involved in this section in an electrolyte solution of 1.5 M vanadium ions and 3 M H2SO4 at a current density of 150 mA/cm2. The picture behind shows the GF@CeO2-x ALD deposited. Furthermore, the slower kinetics of the VRFB positive reaction (2.18) as it is a more complex reaction comparatively to the one electron transfer for the negative VRFBs reaction. Accompanied by the side reactions in the positive half-cell, diminishes the overall battery performance due to a kinetic overpotential that lowers the voltage efficiency of the battery as well as the capacity. For that purpose Ceria is deposited over the positive electrode material, which is a cheap well-known material, in addition to its standard potential and faster kinetics in acidic solution, E0 = 1.72 V, acts avoiding Oxygen Evolution Reaction (OER) during the battery cycling by shifting OER to larger potentials. Moreover, CeO2 and specially, CeO2-x, also enhance the electrocatalytic properties among cycling as a consequence of the electro active oxygen groups, formed on the electrode’s surface. GF@TiO2:H (-) vs. GF@CeO2 (+) GF@TiO2:H (-) vs. GF@CeO2-x (+) 150 mA/cm2 E/V 132PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |