
PDF Publication Title:
Text from PDF Page: 139
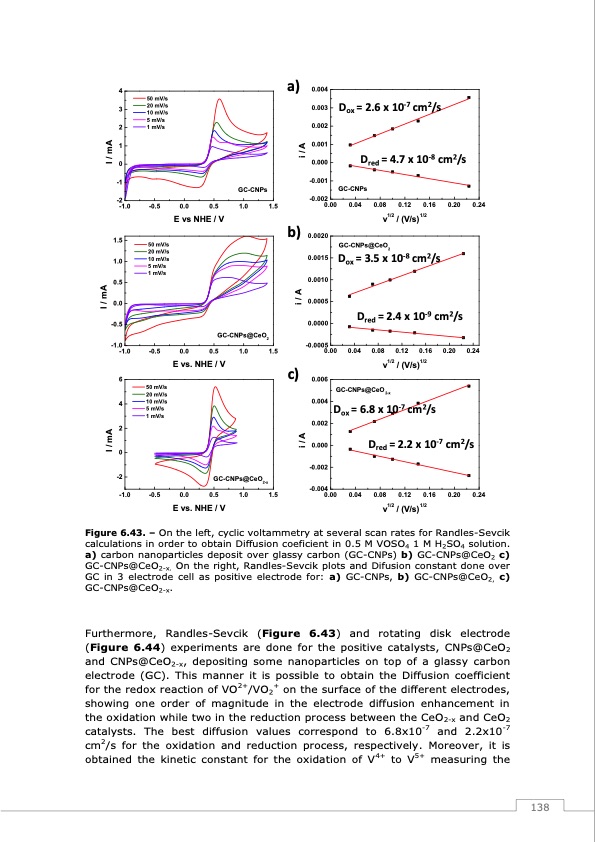
4 3 2 1 0 -1 -2 -1.0 1.5 1.0 0.5 0.0 -0.5 -1.0 -1.0 6 4 2 0 -2 -1.0 50 mV/s 20 mV/s 10 mV/s 5 mV/s 1 mV/s -0.5 50 mV/s 20 mV/s 10 mV/s 5 mV/s 1 mV/s -0.5 50 mV/s 20 mV/s 10 mV/s 5 mV/s 1 mV/s -0.5 0.004 0.003 0.002 0.001 0.000 Dox = 2.6 x 10-7 cm2/s Dred = 4.7 x 10-8 cm2/s 0.0 0.5 E vs NHE / V GC-CNPs 1.0 1.5 GC-CNPs 0.04 0.08 0.12 0.16 0.20 0.24 0.0 0.5 1.0 1.5 0.04 0.08 0.12 0.16 0.20 0.24 E vs. NHE / V c) v1/2 / (V/s)1/2 GC-CNPs@CeO 2-x Dox = 6.8 x 10-7 cm2/s Dred = 2.2 x 10-7 cm2/s 0.0 GC-CNPs@CeO2-x 0.5 1.0 1.5 0.006 0.004 0.002 0.000 -0.002 -0.004 0.00 0.04 0.08 0.12 0.16 v1/2 / (V/s)1/2 0.20 0.24 E vs. NHE / V GC-CNPs@CeO2 a) -0.001 -0.002 0.00 0.0020 0.0015 0.0010 0.0005 0.0000 -0.0005 0.00 b) v1/2 / (V/s)1/2 GC-CNPs@CeO2 -8 2 Dox=3.5x10 cm /s Dred = 2.4 x 10-9 cm2/s I / mA I / mA I / mA i/A i/A i/A Figure 6.43. – On the left, cyclic voltammetry at several scan rates for Randles-Sevcik calculations in order to obtain Diffusion coeficient in 0.5 M VOSO4 1 M H2SO4 solution. a) carbon nanoparticles deposit over glassy carbon (GC-CNPs) b) GC-CNPs@CeO2 c) GC-CNPs@CeO2-x. On the right, Randles-Sevcik plots and Difusion constant done over GC in 3 electrode cell as positive electrode for: a) GC-CNPs, b) GC-CNPs@CeO2, c) GC-CNPs@CeO2-x. Furthermore, Randles-Sevcik (Figure 6.43) and rotating disk electrode (Figure 6.44) experiments are done for the positive catalysts, CNPs@CeO2 and CNPs@CeO2-x, depositing some nanoparticles on top of a glassy carbon electrode (GC). This manner it is possible to obtain the Diffusion coefficient for the redox reaction of VO2+/VO2+ on the surface of the different electrodes, showing one order of magnitude in the electrode diffusion enhancement in the oxidation while two in the reduction process between the CeO2-x and CeO2 catalysts. The best diffusion values correspond to 6.8x10-7 and 2.2x10-7 cm2/s for the oxidation and reduction process, respectively. Moreover, it is obtained the kinetic constant for the oxidation of V4+ to V5+ measuring the 138PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |