
PDF Publication Title:
Text from PDF Page: 147
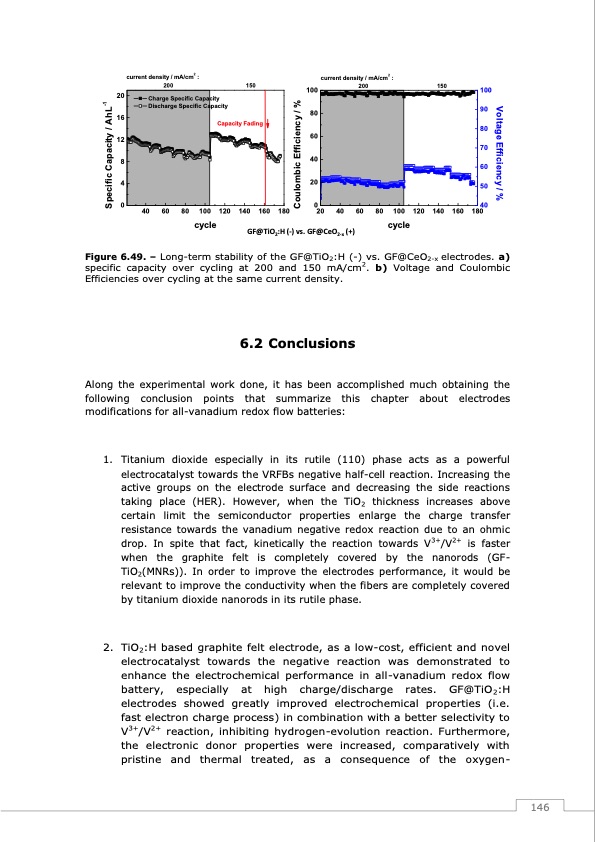
current density / mA/cm2 : 200 150 current density / mA/cm2 : 100 200 80 60 40 20 150 100 90 80 70 60 50 Charge Specific Capacity Discharge Specific Capacity Capacity Fading 20 16 12 8 4 0 40 60 80 100 120 140 160 180 0 40 20 40 60 80 100 120 140 160 180 cycle GF@TiO2:H (-) vs. GF@CeO2-x (+) cycle Figure 6.49. – Long-term stability of the GF@TiO2:H (-)2vs. GF@CeO2-x electrodes. a) specific capacity over cycling at 200 and 150 mA/cm . b) Voltage and Coulombic Efficiencies over cycling at the same current density. 6.2 Conclusions Along the experimental work done, it has been accomplished much obtaining the following conclusion points that summarize this chapter about electrodes modifications for all-vanadium redox flow batteries: 1. Titanium dioxide especially in its rutile (110) phase acts as a powerful electrocatalyst towards the VRFBs negative half-cell reaction. Increasing the active groups on the electrode surface and decreasing the side reactions taking place (HER). However, when the TiO2 thickness increases above certain limit the semiconductor properties enlarge the charge transfer resistance towards the vanadium negative redox reaction due to an ohmic drop. In spite that fact, kinetically the reaction towards V3+/V2+ is faster when the graphite felt is completely covered by the nanorods (GF- TiO2(MNRs)). In order to improve the electrodes performance, it would be relevant to improve the conductivity when the fibers are completely covered by titanium dioxide nanorods in its rutile phase. 2. TiO2:H based graphite felt electrode, as a low-cost, efficient and novel electrocatalyst towards the negative reaction was demonstrated to enhance the electrochemical performance in all-vanadium redox flow battery, especially at high charge/discharge rates. GF@TiO2:H electrodes showed greatly improved electrochemical properties (i.e. fast electron charge process) in combination with a better selectivity to V3+/V2+ reaction, inhibiting hydrogen-evolution reaction. Furthermore, the electronic donor properties were increased, comparatively with pristine and thermal treated, as a consequence of the oxygen- 146 Specific Capacity / AhL-1 Coulombic Efficiency / % Voltage Efficiency / %PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |