
PDF Publication Title:
Text from PDF Page: 154
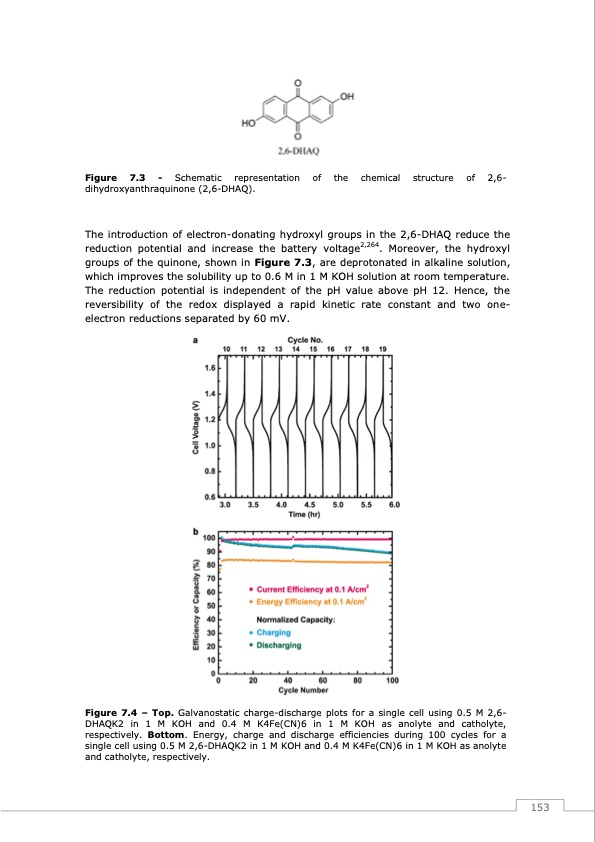
Figure 7.3 - Schematic representation of the chemical structure of 2,6- dihydroxyanthraquinone (2,6-DHAQ). The introduction of electron-donating hydroxyl groups in the 2,6-DHAQ reduce the reduction potential and increase the battery voltage2,264. Moreover, the hydroxyl groups of the quinone, shown in Figure 7.3, are deprotonated in alkaline solution, which improves the solubility up to 0.6 M in 1 M KOH solution at room temperature. The reduction potential is independent of the pH value above pH 12. Hence, the reversibility of the redox displayed a rapid kinetic rate constant and two one- electron reductions separated by 60 mV. Figure 7.4 – Top. Galvanostatic charge-discharge plots for a single cell using 0.5 M 2,6- DHAQK2 in 1 M KOH and 0.4 M K4Fe(CN)6 in 1 M KOH as anolyte and catholyte, respectively. Bottom. Energy, charge and discharge efficiencies during 100 cycles for a single cell using 0.5 M 2,6-DHAQK2 in 1 M KOH and 0.4 M K4Fe(CN)6 in 1 M KOH as anolyte and catholyte, respectively. 153PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |