
PDF Publication Title:
Text from PDF Page: 157
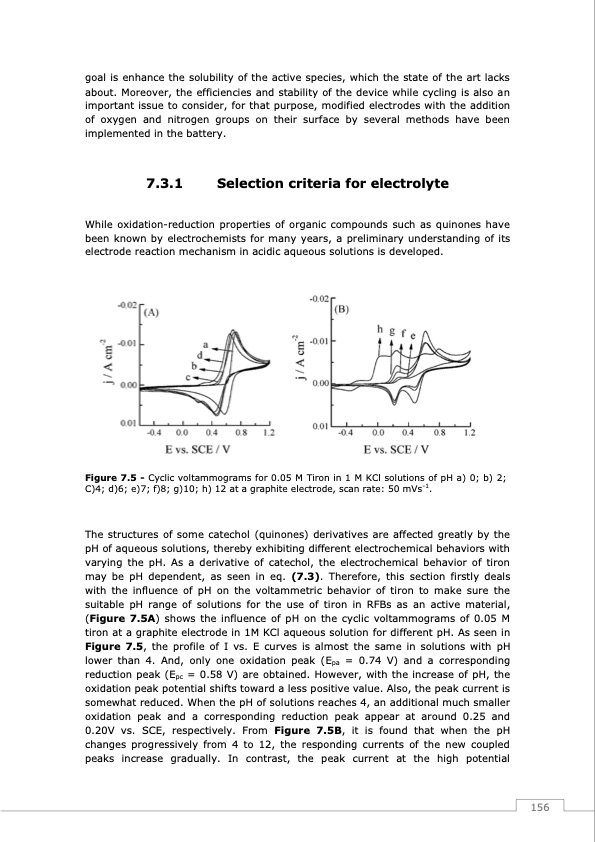
goal is enhance the solubility of the active species, which the state of the art lacks about. Moreover, the efficiencies and stability of the device while cycling is also an important issue to consider, for that purpose, modified electrodes with the addition of oxygen and nitrogen groups on their surface by several methods have been implemented in the battery. 7.3.1 Selection criteria for electrolyte While oxidation-reduction properties of organic compounds such as quinones have been known by electrochemists for many years, a preliminary understanding of its electrode reaction mechanism in acidic aqueous solutions is developed. Figure 7.5 - Cyclic voltammograms for 0.05 M Tiron in 1 M KCl solutions of pH a) 0; b) 2; C)4; d)6; e)7; f)8; g)10; h) 12 at a graphite electrode, scan rate: 50 mVs-1. The structures of some catechol (quinones) derivatives are affected greatly by the pH of aqueous solutions, thereby exhibiting different electrochemical behaviors with varying the pH. As a derivative of catechol, the electrochemical behavior of tiron may be pH dependent, as seen in eq. (7.3). Therefore, this section firstly deals with the influence of pH on the voltammetric behavior of tiron to make sure the suitable pH range of solutions for the use of tiron in RFBs as an active material, (Figure 7.5A) shows the influence of pH on the cyclic voltammograms of 0.05 M tiron at a graphite electrode in 1M KCl aqueous solution for different pH. As seen in Figure 7.5, the profile of I vs. E curves is almost the same in solutions with pH lower than 4. And, only one oxidation peak (Epa = 0.74 V) and a corresponding reduction peak (Epc = 0.58 V) are obtained. However, with the increase of pH, the oxidation peak potential shifts toward a less positive value. Also, the peak current is somewhat reduced. When the pH of solutions reaches 4, an additional much smaller oxidation peak and a corresponding reduction peak appear at around 0.25 and 0.20V vs. SCE, respectively. From Figure 7.5B, it is found that when the pH changes progressively from 4 to 12, the responding currents of the new coupled peaks increase gradually. In contrast, the peak current at the high potential 156PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |