
PDF Publication Title:
Text from PDF Page: 161
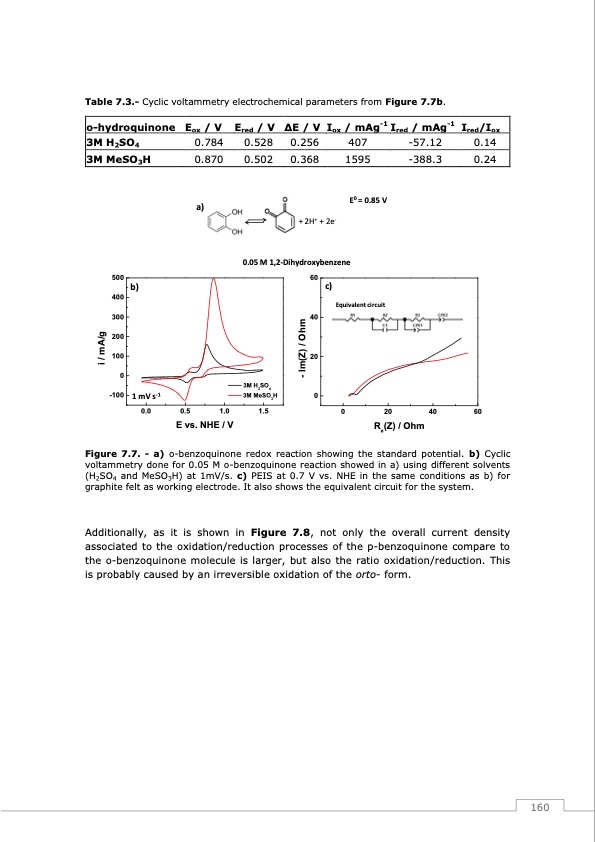
Table 7.3.- Cyclic voltammetry electrochemical parameters from Figure 7.7b. o-hydroquinone Eox / V Ered / V ΔE / V Iox / mAg-1 Ired / mAg-1 Ired/Iox 3M H2SO4 3M MeSO3H 500 400 300 200 100 0 -100 0.784 0.870 a) 0.528 0.256 407 -57.12 0.14 0.502 0.368 1595 -388.3 0.24 E0 = 0.85 V 0.05 M 1,2-Dihydroxybenzene b) 1 mV s-1 60 40 20 3M H2SO4 3M MeSO3H 0 c) i / mA/g - Im(Z) / Ohm Figure 7.7. - a) o-benzoquinone redox reaction showing the standard potential. b) Cyclic voltammetry done for 0.05 M o-benzoquinone reaction showed in a) using different solvents (H2SO4 and MeSO3H) at 1mV/s. c) PEIS at 0.7 V vs. NHE in the same conditions as b) for graphite felt as working electrode. It also shows the equivalent circuit for the system. Additionally, as it is shown in Figure 7.8, not only the overall current density associated to the oxidation/reduction processes of the p-benzoquinone compare to the o-benzoquinone molecule is larger, but also the ratio oxidation/reduction. This is probably caused by an irreversible oxidation of the orto- form. + 2H+ + 2e- Equivalent circuit 0.0 0.5 1.0 1.5 E vs. NHE / V 0 20 Re(Z) / Ohm 40 60 160PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |