
PDF Publication Title:
Text from PDF Page: 174
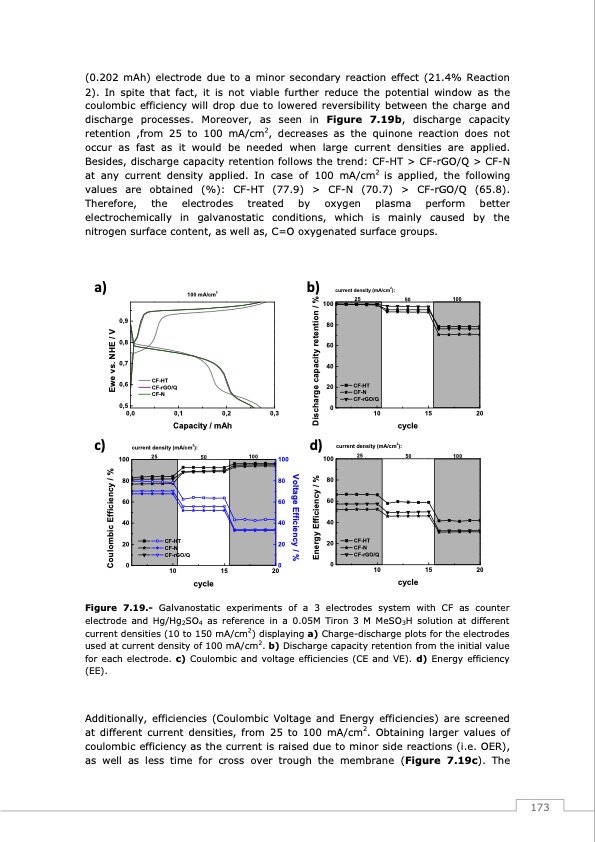
(0.202 mAh) electrode due to a minor secondary reaction effect (21.4% Reaction 2). In spite that fact, it is not viable further reduce the potential window as the coulombic efficiency will drop due to lowered reversibility between the charge and discharge processes. Moreover, as seen in Figure 7.19b, discharge capacity retention ,from 25 to 100 mA/cm2, decreases as the quinone reaction does not occur as fast as it would be needed when large current densities are applied. Besides, discharge capacity retention follows the trend: CF-HT > CF-rGO/Q > CF-N at any current density applied. In case of 100 mA/cm2 is applied, the following values are obtained (%): CF-HT (77.9) > CF-N (70.7) > CF-rGO/Q (65.8). Therefore, the electrodes treated by oxygen plasma perform better electrochemically in galvanostatic conditions, which is mainly caused by the nitrogen surface content, as well as, C=O oxygenated surface groups. a) 100 mA/cm2 b) current density (mA/cm2): 25 10 100 80 60 40 20 0 50 cycle 100 CF-HT CF-N CF-rGO/Q 0,9 0,8 0,7 0,6 0,5 0,0 0,1 0,2 0,3 100 100 15 20 CF-HT CF-rGO/Q CF-N c) Capacity / mAh current density (mA/cm2): 100 25 50 d) current density (mA/cm2): 25 50 100 100 CF-HT CF-N CF-rGO/Q CF-HT CF-N CF-rGO/Q 80 8080 60 6060 40 4040 20 2020 000 10 15 20 cycle 10 15 20 cycle Figure 7.19.- Galvanostatic experiments of a 3 electrodes system with CF as counter electrode and Hg/Hg2SO4 as reference in a 0.05M Tiron 3 M MeSO3H solution at different current densities (10 to 150 mA/cm2) displaying a) Charge-discharge plots for the electrodes used at current density of 100 mA/cm2. b) Discharge capacity retention from the initial value for each electrode. c) Coulombic and voltage efficiencies (CE and VE). d) Energy efficiency (EE). Additionally, efficiencies (Coulombic Voltage and Energy efficiencies) are screened at different current densities, from 25 to 100 mA/cm2. Obtaining larger values of coulombic efficiency as the current is raised due to minor side reactions (i.e. OER), as well as less time for cross over trough the membrane (Figure 7.19c). The 173 Voltage Efficiency / % Coulombic Efficiency / % Ewe vs. NHE / V Energy Efficiency / % Discharge capacity retention / %PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |