
PDF Publication Title:
Text from PDF Page: 235
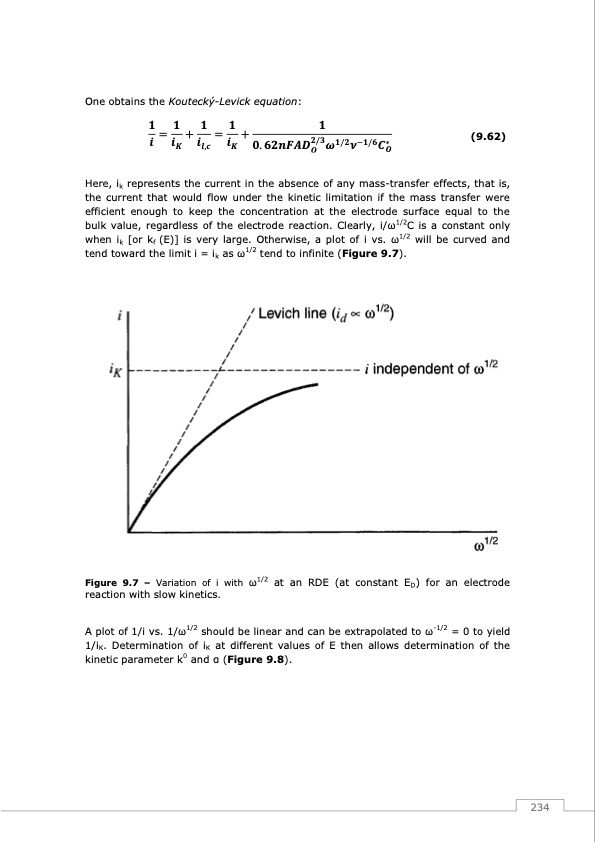
One obtains the Koutecký-Levick equation: Here, ik represents the current in the absence of any mass-transfer effects, that is, the current that would flow under the kinetic limitation if the mass transfer were efficient enough to keep the concentration at the electrode surface equal to the bulk value, regardless of the electrode reaction. Clearly, i/ω1/2C is a constant only when ik [or kf (E)] is very large. Otherwise, a plot of i vs. ω1/2 will be curved and tend toward the limit i = ik as ω1/2 tend to infinite (Figure 9.7). Figure 9.7 – Variation of i with ω1/2 at an RDE (at constant ED) for an electrode reaction with slow kinetics. A plot of 1/i vs. 1/ω1/2 should be linear and can be extrapolated to ω-1/2 = 0 to yield 1/iK. Determination of iK at different values of E then allows determination of the kinetic parameter k0 and α (Figure 9.8). (9.62) 234PDF Image | Redox Flow Batteries Vanadium to Earth Quinones
PDF Search Title:
Redox Flow Batteries Vanadium to Earth QuinonesOriginal File Name Searched:
FJVG_TESIS.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |