
PDF Publication Title:
Text from PDF Page: 023
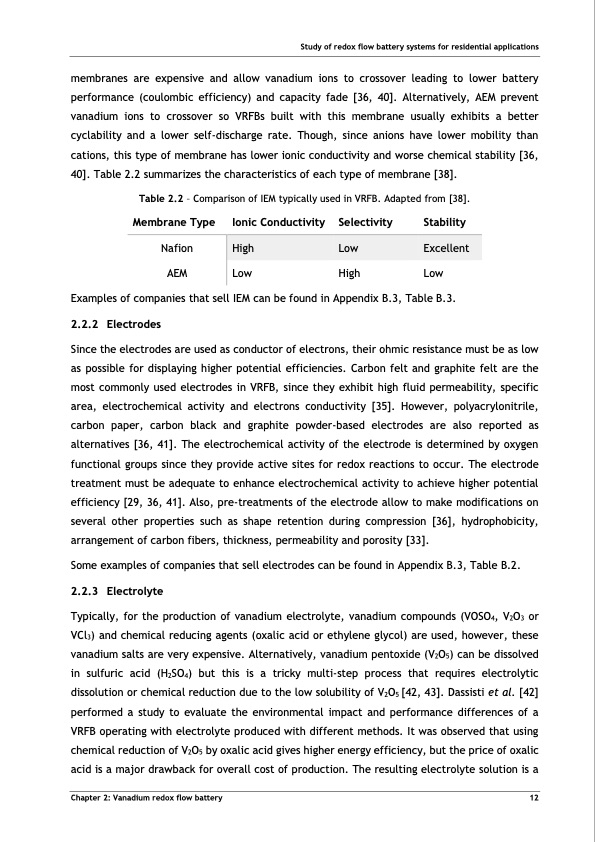
Study of redox flow battery systems for residential applications membranes are expensive and allow vanadium ions to crossover leading to lower battery performance (coulombic efficiency) and capacity fade [36, 40]. Alternatively, AEM prevent vanadium ions to crossover so VRFBs built with this membrane usually exhibits a better cyclability and a lower self-discharge rate. Though, since anions have lower mobility than cations, this type of membrane has lower ionic conductivity and worse chemical stability [36, 40]. Table 2.2 summarizes the characteristics of each type of membrane [38]. Table 2.2 – Comparison of IEM typically used in VRFB. Adapted from [38]. Low High Low Examples of companies that sell IEM can be found in Appendix B.3, Table B.3. 2.2.2 Electrodes Since the electrodes are used as conductor of electrons, their ohmic resistance must be as low as possible for displaying higher potential efficiencies. Carbon felt and graphite felt are the most commonly used electrodes in VRFB, since they exhibit high fluid permeability, specific area, electrochemical activity and electrons conductivity [35]. However, polyacrylonitrile, carbon paper, carbon black and graphite powder-based electrodes are also reported as alternatives [36, 41]. The electrochemical activity of the electrode is determined by oxygen functional groups since they provide active sites for redox reactions to occur. The electrode treatment must be adequate to enhance electrochemical activity to achieve higher potential efficiency [29, 36, 41]. Also, pre-treatments of the electrode allow to make modifications on several other properties such as shape retention during compression [36], hydrophobicity, arrangement of carbon fibers, thickness, permeability and porosity [33]. Some examples of companies that sell electrodes can be found in Appendix B.3, Table B.2. 2.2.3 Electrolyte Typically, for the production of vanadium electrolyte, vanadium compounds (VOSO4, V2O3 or VCl3) and chemical reducing agents (oxalic acid or ethylene glycol) are used, however, these vanadium salts are very expensive. Alternatively, vanadium pentoxide (V2O5) can be dissolved in sulfuric acid (H2SO4) but this is a tricky multi-step process that requires electrolytic dissolution or chemical reduction due to the low solubility of V2O5 [42, 43]. Dassisti et al. [42] performed a study to evaluate the environmental impact and performance differences of a VRFB operating with electrolyte produced with different methods. It was observed that using chemical reduction of V2O5 by oxalic acid gives higher energy efficiency, but the price of oxalic acid is a major drawback for overall cost of production. The resulting electrolyte solution is a Membrane Type Ionic Conductivity Selectivity Stability Nafion High Low Excellent AEM Chapter 2: Vanadium redox flow battery 12PDF Image | Tubular Vanadium Air Redox‐flow battery
PDF Search Title:
Tubular Vanadium Air Redox‐flow batteryOriginal File Name Searched:
204521.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |