
PDF Publication Title:
Text from PDF Page: 029
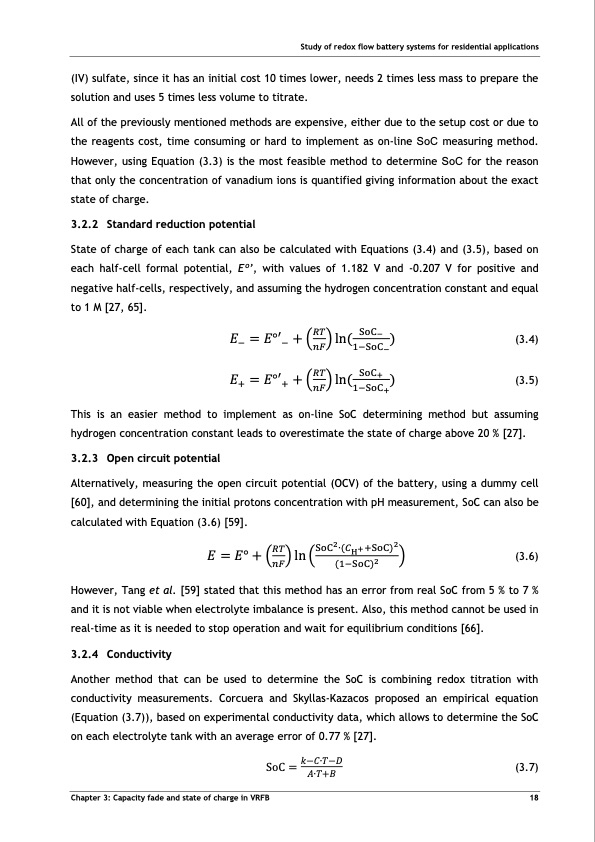
Study of redox flow battery systems for residential applications (IV) sulfate, since it has an initial cost 10 times lower, needs 2 times less mass to prepare the solution and uses 5 times less volume to titrate. All of the previously mentioned methods are expensive, either due to the setup cost or due to the reagents cost, time consuming or hard to implement as on-line SoC measuring method. However, using Equation (3.3) is the most feasible method to determine SoC for the reason that only the concentration of vanadium ions is quantified giving information about the exact state of charge. 3.2.2 Standard reduction potential State of charge of each tank can also be calculated with Equations (3.4) and (3.5), based on each half-cell formal potential, Eo’, with values of 1.182 V and -0.207 V for positive and negative half-cells, respectively, and assuming the hydrogen concentration constant and equal to 1 M [27, 65]. 𝐸−=𝐸°′−+(𝑅𝑇)ln(SoC− ) (3.4) 𝑛𝐹 1−SoC− 𝐸+=𝐸°′++(𝑅𝑇)ln(SoC+ ) (3.5) 𝑛𝐹 1−SoC+ This is an easier method to implement as on-line SoC determining method but assuming hydrogen concentration constant leads to overestimate the state of charge above 20 % [27]. 3.2.3 Open circuit potential Alternatively, measuring the open circuit potential (OCV) of the battery, using a dummy cell [60], and determining the initial protons concentration with pH measurement, SoC can also be calculated with Equation (3.6) [59]. 𝑅𝑇 SoC2∙(𝐶H++SoC)2 𝐸 = 𝐸° + (𝑛𝐹) ln ( (1−SoC)2 ) (3.6) However, Tang et al. [59] stated that this method has an error from real SoC from 5 % to 7 % and it is not viable when electrolyte imbalance is present. Also, this method cannot be used in real-time as it is needed to stop operation and wait for equilibrium conditions [66]. 3.2.4 Conductivity Another method that can be used to determine the SoC is combining redox titration with conductivity measurements. Corcuera and Skyllas-Kazacos proposed an empirical equation (Equation (3.7)), based on experimental conductivity data, which allows to determine the SoC on each electrolyte tank with an average error of 0.77 % [27]. SoC = 𝑘−𝐶∙𝑇−𝐷 (3.7) 𝐴∙𝑇+𝐵 Chapter 3: Capacity fade and state of charge in VRFB 18PDF Image | Tubular Vanadium Air Redox‐flow battery
PDF Search Title:
Tubular Vanadium Air Redox‐flow batteryOriginal File Name Searched:
204521.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |