
PDF Publication Title:
Text from PDF Page: 037
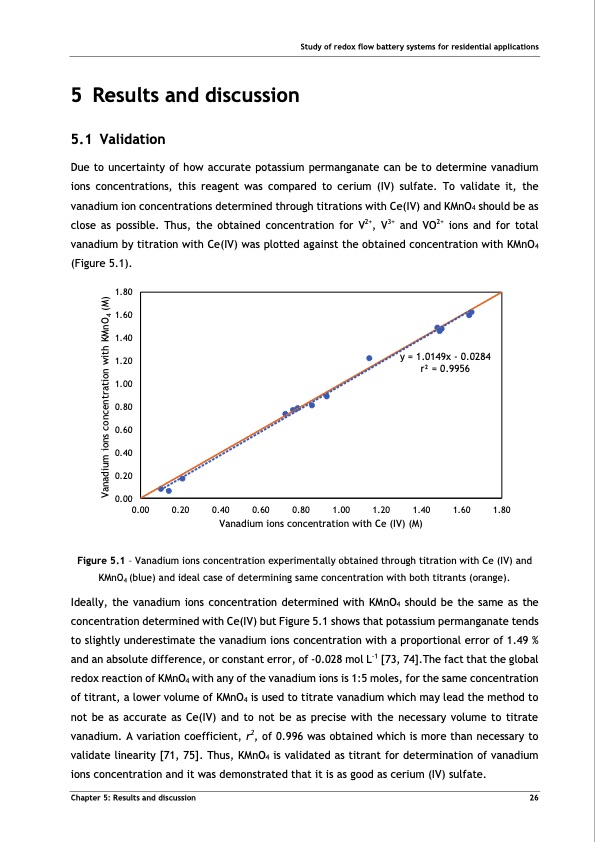
Study of redox flow battery systems for residential applications 5 Results and discussion 5.1 Validation Due to uncertainty of how accurate potassium permanganate can be to determine vanadium ions concentrations, this reagent was compared to cerium (IV) sulfate. To validate it, the vanadium ion concentrations determined through titrations with Ce(IV) and KMnO4 should be as close as possible. Thus, the obtained concentration for V2+, V3+ and VO2+ ions and for total vanadium by titration with Ce(IV) was plotted against the obtained concentration with KMnO4 (Figure 5.1). 1.80 1.60 1.40 1.20 1.00 0.80 0.60 0.40 0.20 0.00 0.00 0.20 Figure 5.1 – Vanadium ions concentration experimentally obtained through titration with Ce (IV) and KMnO4 (blue) and ideal case of determining same concentration with both titrants (orange). Ideally, the vanadium ions concentration determined with KMnO4 should be the same as the concentration determined with Ce(IV) but Figure 5.1 shows that potassium permanganate tends to slightly underestimate the vanadium ions concentration with a proportional error of 1.49 % and an absolute difference, or constant error, of -0.028 mol L-1 [73, 74].The fact that the global redox reaction of KMnO4 with any of the vanadium ions is 1:5 moles, for the same concentration of titrant, a lower volume of KMnO4 is used to titrate vanadium which may lead the method to not be as accurate as Ce(IV) and to not be as precise with the necessary volume to titrate vanadium. A variation coefficient, r2, of 0.996 was obtained which is more than necessary to validate linearity [71, 75]. Thus, KMnO4 is validated as titrant for determination of vanadium ions concentration and it was demonstrated that it is as good as cerium (IV) sulfate. y = 1.0149x - 0.0284 r2 = 0.9956 0.40 0.60 Vanadium ions concentration with Ce (IV) (M) 0.80 1.00 1.20 1.40 1.60 1.80 Chapter 5: Results and discussion 26 Vanadium ions concentration with KMnO4 (M)PDF Image | Tubular Vanadium Air Redox‐flow battery
PDF Search Title:
Tubular Vanadium Air Redox‐flow batteryOriginal File Name Searched:
204521.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |