
PDF Publication Title:
Text from PDF Page: 039
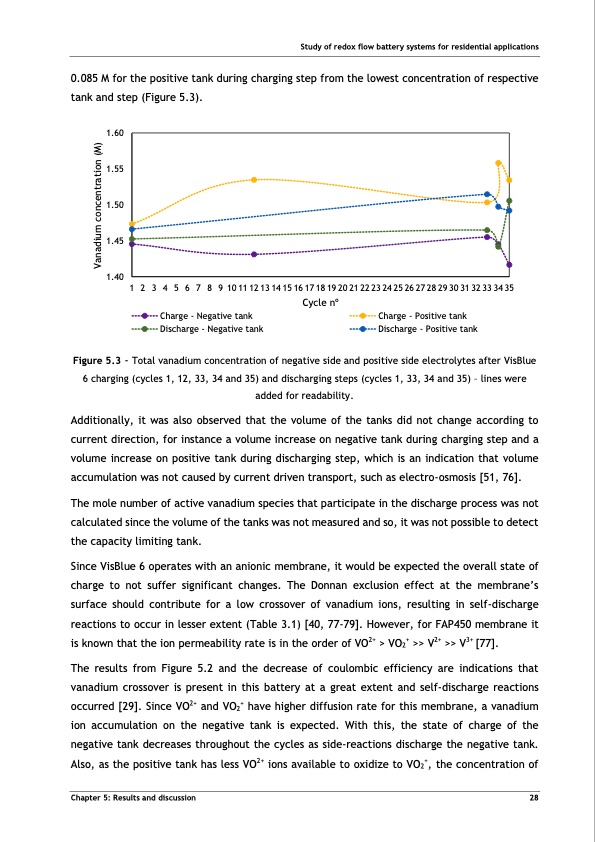
Study of redox flow battery systems for residential applications 0.085 M for the positive tank during charging step from the lowest concentration of respective tank and step (Figure 5.3). 1.60 1.55 1.50 1.45 1.40 1 2 3 4 5 6 7 8 9 1011121314151617181920212223242526272829303132333435 Cycle no Charge - Negative tank Discharge - Negative tank Charge - Positive tank Discharge - Positive tank Figure 5.3 - Total vanadium concentration of negative side and positive side electrolytes after VisBlue 6 charging (cycles 1, 12, 33, 34 and 35) and discharging steps (cycles 1, 33, 34 and 35) – lines were added for readability. Additionally, it was also observed that the volume of the tanks did not change according to current direction, for instance a volume increase on negative tank during charging step and a volume increase on positive tank during discharging step, which is an indication that volume accumulation was not caused by current driven transport, such as electro-osmosis [51, 76]. The mole number of active vanadium species that participate in the discharge process was not calculated since the volume of the tanks was not measured and so, it was not possible to detect the capacity limiting tank. Since VisBlue 6 operates with an anionic membrane, it would be expected the overall state of charge to not suffer significant changes. The Donnan exclusion effect at the membrane’s surface should contribute for a low crossover of vanadium ions, resulting in self-discharge reactions to occur in lesser extent (Table 3.1) [40, 77-79]. However, for FAP450 membrane it is known that the ion permeability rate is in the order of VO2+ > VO2+ >> V2+ >> V3+ [77]. The results from Figure 5.2 and the decrease of coulombic efficiency are indications that vanadium crossover is present in this battery at a great extent and self-discharge reactions occurred [29]. Since VO2+ and VO2+ have higher diffusion rate for this membrane, a vanadium ion accumulation on the negative tank is expected. With this, the state of charge of the negative tank decreases throughout the cycles as side-reactions discharge the negative tank. Also, as the positive tank has less VO2+ ions available to oxidize to VO2+, the concentration of Chapter 5: Results and discussion 28 Vanadium concentration (M)PDF Image | Tubular Vanadium Air Redox‐flow battery
PDF Search Title:
Tubular Vanadium Air Redox‐flow batteryOriginal File Name Searched:
204521.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |