
PDF Publication Title:
Text from PDF Page: 003
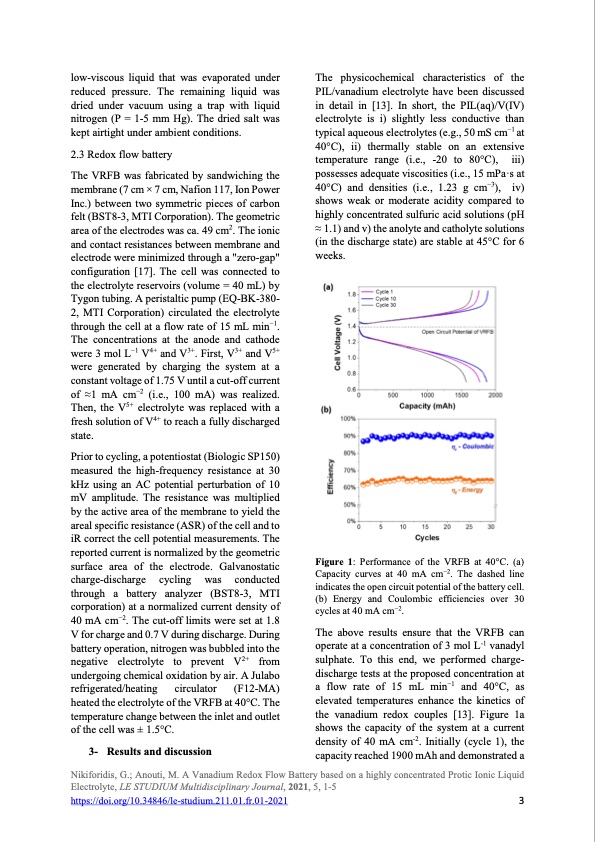
low-viscous liquid that was evaporated under reduced pressure. The remaining liquid was dried under vacuum using a trap with liquid nitrogen (P = 1-5 mm Hg). The dried salt was kept airtight under ambient conditions. 2.3 Redox flow battery The VRFB was fabricated by sandwiching the membrane (7 cm × 7 cm, Nafion 117, Ion Power Inc.) between two symmetric pieces of carbon felt (BST8-3, MTI Corporation). The geometric area of the electrodes was ca. 49 cm2. The ionic and contact resistances between membrane and electrode were minimized through a "zero-gap" configuration [17]. The cell was connected to the electrolyte reservoirs (volume = 40 mL) by Tygon tubing. A peristaltic pump (EQ-BK-380- 2, MTI Corporation) circulated the electrolyte through the cell at a flow rate of 15 mL min−1. The concentrations at the anode and cathode were 3 mol L−1 V4+ and V3+. First, V3+ and V5+ were generated by charging the system at a constant voltage of 1.75 V until a cut-off current of ≈1 mA cm−2 (i.e., 100 mA) was realized. Then, the V5+ electrolyte was replaced with a fresh solution of V4+ to reach a fully discharged state. Prior to cycling, a potentiostat (Biologic SP150) measured the high-frequency resistance at 30 kHz using an AC potential perturbation of 10 mV amplitude. The resistance was multiplied by the active area of the membrane to yield the areal specific resistance (ASR) of the cell and to iR correct the cell potential measurements. The reported current is normalized by the geometric surface area of the electrode. Galvanostatic charge-discharge cycling was conducted through a battery analyzer (BST8-3, MTI corporation) at a normalized current density of 40 mA cm−2. The cut-off limits were set at 1.8 V for charge and 0.7 V during discharge. During battery operation, nitrogen was bubbled into the negative electrolyte to prevent V2+ from undergoing chemical oxidation by air. A Julabo refrigerated/heating circulator (F12-MA) heated the electrolyte of the VRFB at 40°C. The temperature change between the inlet and outlet of the cell was ± 1.5°C. 3- Results and discussion The physicochemical characteristics of the PIL/vanadium electrolyte have been discussed in detail in [13]. In short, the PIL(aq)/V(IV) electrolyte is i) slightly less conductive than typical aqueous electrolytes (e.g., 50 mS cm−1 at 40°C), ii) thermally stable on an extensive temperature range (i.e., -20 to 80°C), iii) possesses adequate viscosities (i.e., 15 mPa·s at 40°C) and densities (i.e., 1.23 g cm−3), iv) shows weak or moderate acidity compared to highly concentrated sulfuric acid solutions (pH ≈ 1.1) and v) the anolyte and catholyte solutions (in the discharge state) are stable at 45°C for 6 weeks. Figure 1: Performance of the VRFB at 40°C. (a) Capacity curves at 40 mA cm−2. The dashed line indicates the open circuit potential of the battery cell. (b) Energy and Coulombic efficiencies over 30 cycles at 40 mA cm−2. The above results ensure that the VRFB can operate at a concentration of 3 mol L-1 vanadyl sulphate. To this end, we performed charge- discharge tests at the proposed concentration at a flow rate of 15 mL min−1 and 40°C, as elevated temperatures enhance the kinetics of the vanadium redox couples [13]. Figure 1a shows the capacity of the system at a current density of 40 mA cm-2. Initially (cycle 1), the capacity reached 1900 mAh and demonstrated a Nikiforidis, G.; Anouti, M. A Vanadium Redox Flow Battery based on a highly concentrated Protic Ionic Liquid Electrolyte, LE STUDIUM Multidisciplinary Journal, 2021, 5, 1-5 https://doi.org/10.34846/le-studium.211.01.fr.01-2021 3PDF Image | Vanadium Redox Flow Battery Protic Ionic Liquid Electrolyte
PDF Search Title:
Vanadium Redox Flow Battery Protic Ionic Liquid ElectrolyteOriginal File Name Searched:
nikiforidis_lestudiumjournal.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |