
PDF Publication Title:
Text from PDF Page: 020
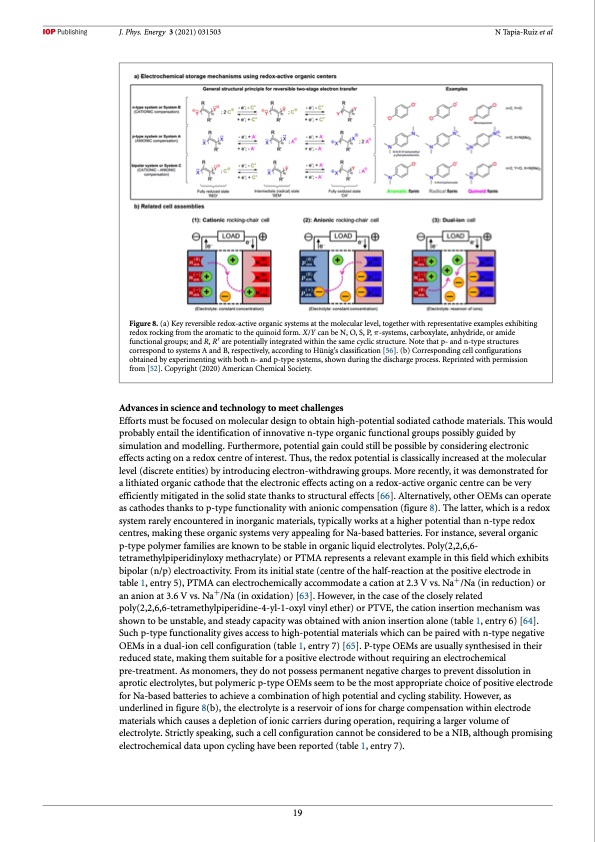
J. Phys. Energy 3 (2021) 031503 N Tapia-Ruiz et al Figure 8. (a) Key reversible redox-active organic systems at the molecular level, together with representative examples exhibiting redox rocking from the aromatic to the quinoid form. X/Y can be N, O, S, P, π-systems, carboxylate, anhydride, or amide functional groups; and R, R′ are potentially integrated within the same cyclic structure. Note that p- and n-type structures correspond to systems A and B, respectively, according to Hünig’s classification [56]. (b) Corresponding cell configurations obtained by experimenting with both n- and p-type systems, shown during the discharge process. Reprinted with permission from [52]. Copyright (2020) American Chemical Society. Advances in science and technology to meet challenges Efforts must be focused on molecular design to obtain high-potential sodiated cathode materials. This would probably entail the identification of innovative n-type organic functional groups possibly guided by simulation and modelling. Furthermore, potential gain could still be possible by considering electronic effects acting on a redox centre of interest. Thus, the redox potential is classically increased at the molecular level (discrete entities) by introducing electron-withdrawing groups. More recently, it was demonstrated for a lithiated organic cathode that the electronic effects acting on a redox-active organic centre can be very efficiently mitigated in the solid state thanks to structural effects [66]. Alternatively, other OEMs can operate as cathodes thanks to p-type functionality with anionic compensation (figure 8). The latter, which is a redox system rarely encountered in inorganic materials, typically works at a higher potential than n-type redox centres, making these organic systems very appealing for Na-based batteries. For instance, several organic p-type polymer families are known to be stable in organic liquid electrolytes. Poly(2,2,6,6- tetramethylpiperidinyloxy methacrylate) or PTMA represents a relevant example in this field which exhibits bipolar (n/p) electroactivity. From its initial state (centre of the half-reaction at the positive electrode in table 1, entry 5), PTMA can electrochemically accommodate a cation at 2.3 V vs. Na+/Na (in reduction) or an anion at 3.6 V vs. Na+/Na (in oxidation) [63]. However, in the case of the closely related poly(2,2,6,6-tetramethylpiperidine-4-yl-1-oxyl vinyl ether) or PTVE, the cation insertion mechanism was shown to be unstable, and steady capacity was obtained with anion insertion alone (table 1, entry 6) [64]. Such p-type functionality gives access to high-potential materials which can be paired with n-type negative OEMs in a dual-ion cell configuration (table 1, entry 7) [65]. P-type OEMs are usually synthesised in their reduced state, making them suitable for a positive electrode without requiring an electrochemical pre-treatment. As monomers, they do not possess permanent negative charges to prevent dissolution in aprotic electrolytes, but polymeric p-type OEMs seem to be the most appropriate choice of positive electrode for Na-based batteries to achieve a combination of high potential and cycling stability. However, as underlined in figure 8(b), the electrolyte is a reservoir of ions for charge compensation within electrode materials which causes a depletion of ionic carriers during operation, requiring a larger volume of electrolyte. Strictly speaking, such a cell configuration cannot be considered to be a NIB, although promising electrochemical data upon cycling have been reported (table 1, entry 7). 19PDF Image | 2021 roadmap for sodium-ion batteries
PDF Search Title:
2021 roadmap for sodium-ion batteriesOriginal File Name Searched:
roadmap-sodium-ion-batteries_031503.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |