
PDF Publication Title:
Text from PDF Page: 006
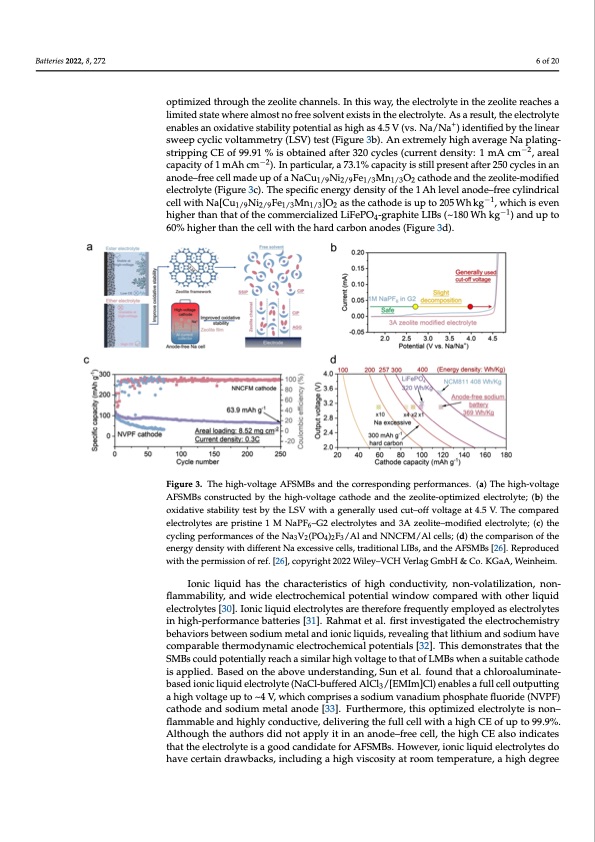
Batteries 2022, 8, 272 enable a dendrite-free Na plating/stripping CE as high as 99% in the Na/Cu cell [25]. To further reach the high reversibility of Na plating/stripping processes while expanding the oxidative stability, Lu et al. rationally designed a beyond−concentrated electrolyte by a 3A zeolite molecular sieve film [26]. As shown in Figure 3a, a high-voltage AFSMB is built 6 of 20 by coating a zeolite film on the Al current collector, where the free solvent in the pure diglyme-based electrolyte will be optimized through the zeolite channels. In this way, the electrolyte in the zeolite reaches a limited state where almost no free solvent exists in the optimized through the zeolite channels. In this way, the electrolyte in the zeolite reaches a electrolyte. As a result, the electrolyte enables an oxidative stability potential as high as limitedstatewhere+ almostnofreesolventexistsintheelectrolyte.Asaresult,theelectrolyte 4.5 V (vs. Na/Na ) identified by the linear sweep cyclic voltammetry (LSV) test (Figure enables an oxidative stability potential as high as 4.5 V (vs. Na/Na+) identified by the linear 3b). An extremely high average Na plating-stripping CE of 99.91 % is obtained after 320 sweep cyclic voltammetry (LSV) test (Figure 3b). An extremely high average Na plating- cycles (current density: 1 mA cm−2, areal capacity of 1 mAh cm−2). In particular, a 73.1% stripping CE of 99.91 % is obtained after 320 cycles (current density: 1 mA cm−2, areal capacity is still present after 250 cycles in an anode−free cell made up of a capacity of 1 mAh cm−2). In particular, a 73.1% capacity is still present after 250 cycles in an NaCu1/9Ni2/9Fe1/3Mn1/3O2 cathode and the zeolite-modified electrolyte (Figure 3c). The spe- anode–free cell made up of a NaCu1/9Ni2/9Fe1/3Mn1/3O2 cathode and the zeolite-modified cific energy density of the 1 Ah level anode−free cylindrical cell with electrolyte (Figure 3c). The specific energy density of the 1 Ah level anode–free cylindrical Na[Cu1/9Ni2/9Fe1/3Mn1/3]O2 as the cathode is up to 205 Wh kg−1, which is even higher than cell with Na[Cu Ni Fe Mn ]O as the cathode is up to 205 Wh kg−1, which is even 1/9 2/9 1/3 1/3 2 −1 thatofthecommercializedLiFePO4-graphiteLIBs(~180Whkg )andupto60%higherthan higher than that of the commercialized LiFePO4-graphite LIBs (~180 Wh kg−1) and up to the cell with the hard carbon anodes (Figure 3d). 60% higher than the cell with the hard carbon anodes (Figure 3d). Figure 3. The high-voltage AFSMBs and the corresponding performances. (a) The high-voltage Figure 3. The high-voltage AFSMBs and the corresponding performances. (a) The high-voltage AFSMBs constructed by the high-voltage cathode and the zeolite-optimized electrolyte; (b) the oxi- AFSMBs constructed by the high-voltage cathode and the zeolite-optimized electrolyte; (b) the dative stability test by the LSV with a generally used cut−off voltage at 4.5 V. The compared elec- oxidative stability test by the LSV with a generally used cut–off voltage at 4.5 V. The compared trolytes are pristine 1 M NaPF6−G2 electrolytes and 3A zeolite−modified electrolyte; (c) the cycling electrolytes are pristine 1 M NaPF6–G2 electrolytes and 3A zeolite–modified electrolyte; (c) the performances of the Na3V2(PO4)2F3/Al and NNCFM/Al cells; (d) the comparison of the energy den- cycling performances of the Na3V2(PO4)2F3/Al and NNCFM/Al cells; (d) the comparison of the sity with different Na excessive cells, traditional LIBs, and the AFSMBs [26]. Reproduced with the energy density with different Na excessive cells, traditional LIBs, and the AFSMBs [26]. Reproduced permission of ref. [26], copyright 2022 Wiley−VCH Verlag GmbH & Co. KGaA, Weinheim. with the permission of ref. [26], copyright 2022 Wiley–VCH Verlag GmbH & Co. KGaA, Weinheim. Ionic liquid has the characteristics of high conductivity, non-volatilization, non-flam- Ionic liquid has the characteristics of high conductivity, non-volatilization, non- mability, and wide electrochemical potential window compared with other liquid electro- flammability, and wide electrochemical potential window compared with other liquid lytes [30]. Ionic liquid electrolytes are therefore frequently employed as electrolytes in electrolytes [30]. Ionic liquid electrolytes are therefore frequently employed as electrolytes high-performance batteries [31]. Rahmat et al. first investigated the electrochemistry be- in high-performance batteries [31]. Rahmat et al. first investigated the electrochemistry haviors between sodium metal and ionic liquids, revealing that lithium and sodium have behaviors between sodium metal and ionic liquids, revealing that lithium and sodium have comparable thermodynamic electrochemical potentials [32]. This demonstrates that the comparable thermodynamic electrochemical potentials [32]. This demonstrates that the SMBs could potentially reach a similar high voltage to that of LMBs when a suitable cath- SMBs could potentially reach a similar high voltage to that of LMBs when a suitable cathode ode is applied. Based on the above understanding, Sun et al. found that a chloroaluminate- is applied. Based on the above understanding, Sun et al. found that a chloroaluminate- based ionic liquid electrolyte (NaCl-buffered AlCl3/[EMIm]Cl) enables a full cell output- based ionic liquid electrolyte (NaCl-buffered AlCl3/[EMIm]Cl) enables a full cell outputting ting a high voltage up to ~4 V, which comprises a sodium vanadium phosphate fluoride a high voltage up to ~4 V, which comprises a sodium vanadium phosphate fluoride (NVPF) cathode and sodium metal anode [33]. Furthermore, this optimized electrolyte is non– flammable and highly conductive, delivering the full cell with a high CE of up to 99.9%. Although the authors did not apply it in an anode–free cell, the high CE also indicates that the electrolyte is a good candidate for AFSMBs. However, ionic liquid electrolytes do have certain drawbacks, including a high viscosity at room temperature, a high degreePDF Image | Anode-Free Rechargeable Sodium-Metal Batteries
PDF Search Title:
Anode-Free Rechargeable Sodium-Metal BatteriesOriginal File Name Searched:
batteries-08-00272.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |