
PDF Publication Title:
Text from PDF Page: 010
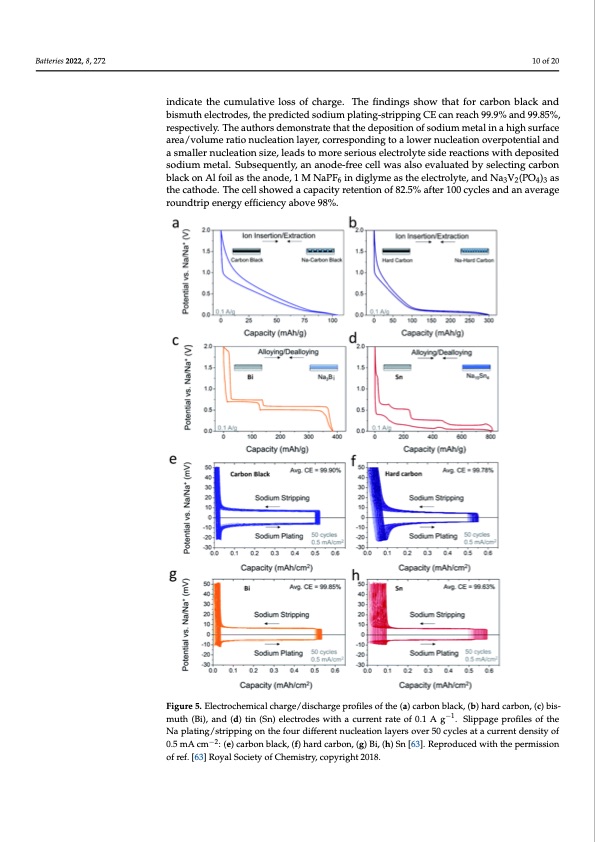
Batteries 2022, 8, 272 10 of 20 Batteries 2022, 8, x FOR PEER REVIEW 10 of 19 indicate the cumulative loss of charge. The findings show that for carbon black and bismuth electrodes, the predicted sodium plating-stripping CE can reach 99.9% and 99.85%, respectively. The authors demonstrate that the deposition of sodium metal in a high surface area/volume ratio nucleation layer, corresponding to a lower nucleation overpotential and area/volume ratio nucleation layer, corresponding to a lower nucleation overpotential and a smaller nucleation size, leads to more serious electrolyte side reactions with deposited a smaller nucleation size, leads to more serious electrolyte side reactions with deposited sodium metal. Subsequently, an anode-free cell was also evaluated by selecting carbon sodium metal. Subsequently, an anode-free cell was also evaluated by selecting carbon black on Al foil as the anode, 1 M NaPF6 in diglyme as the electrolyte, and Na3V2(PO4)3 as black on Al foil as the anode, 1 M NaPF6 in diglyme as the electrolyte, and Na3V2(PO4)3 as the cathode. The cell showed a capacity retention of 82.5% after 100 cycles and an average the cathode. The cell showed a capacity retention of 82.5% after 100 cycles and an average roundtrip energy efficiency above 98%. roundtrip energy efficiency above 98%. Figure 5. Electrochemical charge/discharge profiles of the (a) carbon black, (b) hard carbon, (c) bis- Figure 5. Electrochemical charge/discharge profiles of the (a) carbon black, (b) hard carbon, (c) bis- −1 muth (Bi), and (d) tin (Sn) electrodes with a current rate of 0.1 A g −1 . Slippage profiles of the muth (Bi), and (d) tin (Sn) electrodes with a current rate of 0.1 A g . Slippage profiles of the Na Na plating/stripping on the four different nucleation layers over 50 cycles at a current density of plating/stripping on the four different nucleation layers over 50 cycles at a current density of 0.5 mA 0.5−2 mA cm−2: (e) carbon black, (f) hard carbon, (g) Bi, (h) Sn [63]. Reproduced with the permission cm : (e) carbon black, (f) hard carbon, (g) Bi, (h) Sn [63]. Reproduced with the permission of ref. of ref. [63] Royal Society of Chemistry, copyright 2018. [63] Royal Society of Chemistry, copyright 2018. Based on a comprehensive understanding of the relationship between the properties of the sodium nucleation layer and the plating–striping CE, researchers tend to explore various nu- cleation layers such as the nanocarbon nucleation layer formed on Al current collectors [64], metal−organic frameworks (MOF)-derived copper−-carbon (Cu@C) composite [65], or gra- phitic carbon−coated current collectors [23]. For instance, a nanocarbon nucleation layerPDF Image | Anode-Free Rechargeable Sodium-Metal Batteries
PDF Search Title:
Anode-Free Rechargeable Sodium-Metal BatteriesOriginal File Name Searched:
batteries-08-00272.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |