
PDF Publication Title:
Text from PDF Page: 015
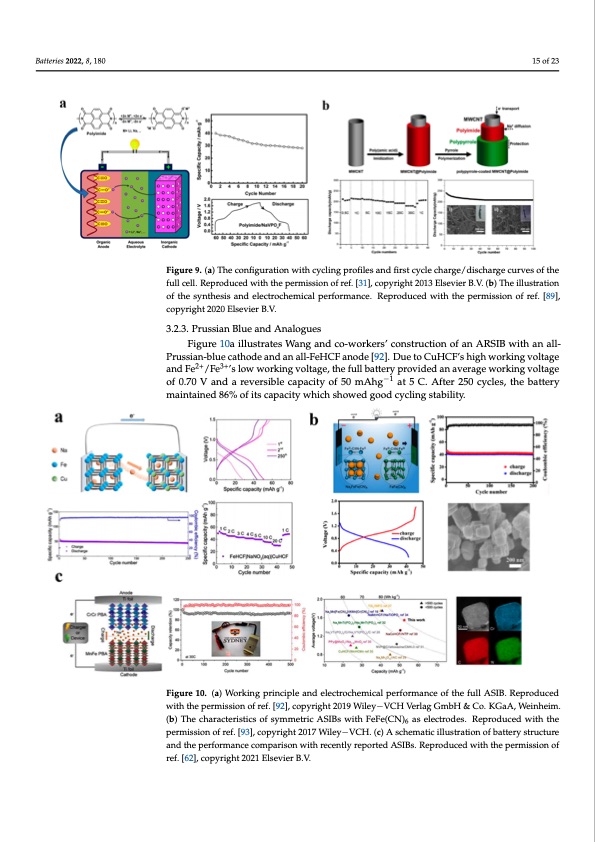
E f a e y n v h [ v k a h 0 a c w h anode material for ASIBs was developed by Kim et al., based on MWCNTs coated w their structural stability and plentiful vanadium chemical flexibility [94]. Anode materials p a , [ a R REVIEW of the synthesis and electrochemical performance. Reproduced with the permission of ref. [89], full cell. Reproduced with the permission of ref. [31], copyright 2013 Elsevier B.V. (b) The illustr Batteries 2022, 8, 180 polypyrrole-doped polyimide nanowires synthesized from pyromellitic dianhyd (PMDA) and 4,4’-oxydianiline (ODA). In Figure 9b, a bicontinuous electron and ion trans pathway allows for a high initial discharge capacity of 234.9 mA h g−1 for the fabric MWCNT@polyimide, which is 83.6% higher than the theoretical value. Afte15r o1f 0230 cycles capacity of the battery retains 209.3 mA h g−1 which is equal to 77.8% of its initial capacity Figure 9. (a) The configuration with cycling profiles and first cycle charge/discharge curves of the Figfurllece9l.l.(Rae)pTrohdeuceodnwfiigthurthaetipoenrmwisistihoncoyfcrleinf.g[3p1]r,ocofiplyersigahntd20f1i3rsEtlsceyvcielreBc.Vh.a(rbg)eT/hdeisilcluhsatragtieoncurves o copyright 2020 Elsevier B.V. 16 of 23 of the synthesis and electrochemical performance. Reproduced with the permission of ref. [89], yright 2020 Elsevier B.V. 3.2.3. Prussian Blue and Analogues Figure 10a illustrates Wang and co-workers’ construction of an ARSIB with an all- Moreover, the capacitors Twheerreeafobrle,ttohreectaoind9u3c.t0i1v%itycapnadcitthyeaefltecrt5ro0c0hceymcliecsalatp3ro0pCeratineds tohfeconducting p Prussian-blue cathode and an all-FeHCF anode [92]. Due to CuHCF’s high working voltage 2+ 3+ mearnsdaFre g/eFneer’aslloywewnohraknincgevdolbtayget,htehierfuπlleblaettcetryopnrocvoidnejudgaantaevdersatgreuwctourkriensg,vwolhtaigche can b coulombic efficiency was close to 100%. As a result of the similar mechanisms by which CrCr of 0.70 V and a reversible capacity of 50 mAhg−1 at 5 C. After 250 cycles, the battery versibly doped and dedoped. Additionally, they can be easily synthesized by appl PBA stores energy, it has the potential to be used as an anode for other types of ASIBs [59]. maintained 86% of its capacity which showed good cycling stability. chemical or electrochemical methods to oxidize the relevant monomer in solution [90] found by Fan and colleagues, PPy nanotubes adhere strongly to the carbon substrate dicating that pyrrole polymerizes homogeneously adjacent to the ammonium vanadate owires. In ASIBs, the performance of PPy nanotubes have been examined by using cyclic ammetry, charge–discharge tests, and rate performance. In light of the battery’s electroc ical characteristics, the results indicate 108.8 mAh g−1 of discharge capacity over 100 cycles 3.2.3. Prussian Blue and Analogues Figure 10a illustrates Wang and co-workers’ construction of an ARSIB with an Prussian-blue cathode and an all-FeHCF anode [92]. Due to CuHCF’s high working age and Fe2+/Fe3+’s low working voltage, the full battery provided an average wor voltage of 0.70 V and a reversible capacity of 50 mAhg−1 at 5 C. After 250 cycles, the bat maintained 86% of its capacity which showed good cycling stability. Furthermore, various benefits are associated with bipolar materials, which are c ble of functioning both as an anode and cathode in ASIBs. Among these benefits are t low sodium cost, and ease of fabrication. For the first time, FeFe(CN)6 nanocubes been made and used as bipolar materials in ASIBs by Junshu Zhang and co-workers shown in Figure 10b, the resulting full cell mainly inherits FeFe(CN)6’s excellent cyc stability and its superior rate capability. A capacity of 32 mAh g−1 was achieved at 2 and at 2 °C it sustained around 97% capacity after 200 cycles which shows a good cap Figure 10. (a) Working principle and electrochemical performance of the full ASIB. Reproduced Figure 10. (a) Working principle and electrochemical performance of the full ASIB. Reproduced retention. Similarly, the cycling performed at 10 C at a fast-charging rate and 2 C at a s with the permission of ref. [92], copyright 2019 Wiley−VCH Verlag GmbH & Co. KGaA, Weinheim. with the permission of ref. [92], copyright 2019 Wiley−VCH Verlag GmbH & Co. KGaA, Weinheim. dis(cbh)aTrhgeicnhgararcatteerisatilcssooafcsyhmiemvetrdicsAimSIBilsawrirthesFuelFtes(C[N93)].aselectrodes. Reproducedwiththe 6 permission of ref. [93], copyright 2017 Wiley−VCH. (c) A schematic illustration of battery structure (b) The characteristics of syAmsmsehtroicwAnSIiBnsFwigituhrFeeF1e0(cC, NC)h6 eans ealencdtrocdoellse.aRgeupersodruepceodrtwedithththaet pcehrr-omium hexa and the performance comparison with recently reported ASIBs. Reproduced with the permission of mission of ref. [93], conpoycrhigrhotm2a0t1e7aWsailneoyd−eVmCaHte.r(ica)lAforscAhSemIBastiecxihlliubsitsraatisopneociffibcacttaepryacsitryuoctfu1r0e8.2mAhg−1, ref. [62], copyright 2021 Elsevier B.V. −1 and the performance caomdipsacrhiasorgnewriattheroefce0n.5tlAy rgepo. ArteddAitiSoInBas.llRye,pCrroCdruPceBdAweixthitbhietspearlmowissrioendoxf potential, w ref. [59], copyright 202i1ncErleseavsieedr Bth.Ve.battery’s voltage. In this study, the complete cell exhibited a capacity of mA kg−1 as well as an energy density of 81.6 Wh kg−1 at a voltage of 1.55 V on aver 3.2.4. Other Materials The sodium vanadium phosphate relatives are a potential series for ASIBs due toPDF Image | Aqueous Rechargeable Sodium-Ion Batteries Hydrogel
PDF Search Title:
Aqueous Rechargeable Sodium-Ion Batteries HydrogelOriginal File Name Searched:
batteries-08-00180-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |