
PDF Publication Title:
Text from PDF Page: 009
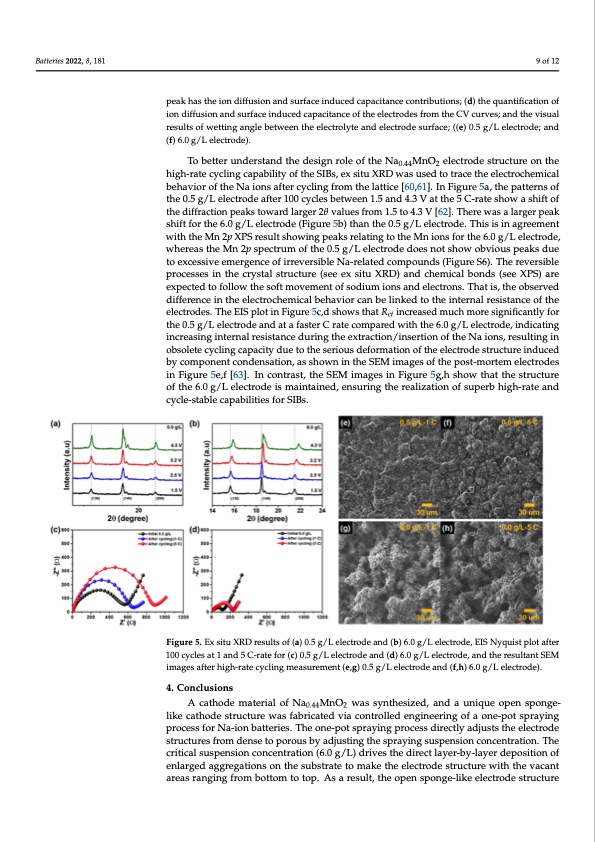
Batteries 2022, 8, 181 9 of 12 Batteries 2022, 8, 181 peak has the ion diffusion and surface induced capacitance contributions; (d) the quantification of ion diffusion and surface induced capacitance of the electrodes from the CV curves; and the visual results of wetting angle between the electrolyte and electrode surface; ((e) 0.5 g/L electrode; and (f) 6.0 g/L electrode). To better understand the design role of the Na0.44MnO2 electrode structure on the high-rate cycling capability of the SIBs, ex situ XRD was used to trace the electrochemical behavior of the Na ions after cycling from the lattice [60,61]. In Figure 5a, the patterns of the 0.5 g/L electrode after 100 cycles between 1.5 and 4.3 V at the 5 C-rate show10a osfh1if3t of the diffraction peaks toward larger 2θ values from 1.5 to 4.3 V [62]. There was a larger peak shift for the 6.0 g/L electrode (Figure 5b) than the 0.5 g/L electrode. This is in agreement with the Mn 2p XPS result showing peaks relating to the Mn ions for the 6.0 g/L electrode, agreement with the Mn 2p XPS result showing peaks relating to the Mn ions for the 6.0 whereas the Mn 2p spectrum of the 0.5 g/L electrode does not show obvious peaks due g/L electrode, whereas the Mn 2p spectrum of the 0.5 g/L electrode does not show obvious topeaxkcessdsuiveetoemexecregsesnivceeomf eirrgevnecresoibflierrNevae-rseilbaltedNac‐ormelaptoeudncdoms (pFoiguunrdesS(F6i)g. uTrheeSr6e)v. Terhseible preovcerssiebsleinprtohcescsreyssitnalthsterucrcytustraels(streuecteuxresi(tsueeXeRxDs)itaunXdRcDh)eamnidcaclhbemonicdasl(bsoenedXsP(sSe)eare exXpPeSc)teadreteoxfpoelclotewdtohefosloloftwmthoevesmoftenmtovfesmodeniutmofisondsiuamndioenlescatnrodnesl.eTcthroantsis.,Tthaetoisb,stehreved doifbfeserervnecde dinifftehreneclecintrtohcehelmecitcraolchbemhaicvailobrechanviboer clainkbeedlitnoktehdetointhterinatel rneaslisrteasniscteanocfethe eloefcthroedeelse.ctTrhodeeEsI.STphleoEtIinSpFliogtuirneF5icg,durseh5ocw,dssthoatwRsthiantcrRecat isnecdremasuecdhmuocrhesmigonreifisciagntiflyi‐for ct thcean0t.l5ygf/orLtehlec0t.r5ogd/eLaenledctartoadefasntedraCt arafatestceormCprartedcowmitpharthede 6w.0ithg/thLee6l.e0ctgr/oLdel,eicntrdoidcaet,ing indicating increasing internal resistance during the extraction/insertion of the Na ions, re‐ increasing internal resistance during the extraction/insertion of the Na ions, resulting in sulting in obsolete cycling capacity due to the serious deformation of the electrode struc‐ obsolete cycling capacity due to the serious deformation of the electrode structure induced ture induced by component condensation, as shown in the SEM images of the post‐mor‐ by component condensation, as shown in the SEM images of the post-mortem electrodes tem electrodes in Figure 5e,f. [63] In contrast, the SEM images in Figure 5g,h show that the in Figure 5e,f [63]. In contrast, the SEM images in Figure 5g,h show that the structure structure of the 6.0 g/L electrode is maintained, ensuring the realization of superb high‐ of the 6.0 g/L electrode is maintained, ensuring the realization of superb high-rate and rate and cycle‐stable capabilities for SIBs. cycle-stable capabilities for SIBs. Figure 5. Ex situ XRD results of (a) 0.5 g/L electrode and (b) 6.0 g/L electrode, EIS Nyquist plot after Figure 5. Ex situ XRD results of (a) 0.5 g/L electrode and (b) 6.0 g/L electrode, EIS Nyquist plot after 100 cycles at 1 and 5 C‐rate for (c) 0.5 g/L electrode and (d) 6.0 g/L electrode, and the resultant SEM 100 cycles at 1 and 5 C-rate for (c) 0.5 g/L electrode and (d) 6.0 g/L electrode, and the resultant SEM images after high‐rate cycling measurement (e,g) 0.5 g/L electrode and (f,h) 6.0 g/L electrode). images after high-rate cycling measurement (e,g) 0.5 g/L electrode and (f,h) 6.0 g/L electrode). 4. Conclusions 4. Conclusions A cathode material of Na0.44MnO2 was synthesized, and a unique open sponge‐like A cathode material of Na0.44MnO2 was synthesized, and a unique open sponge- cathode structure was fabricated via controlled engineering of a one‐pot spraying process like cathode structure was fabricated via controlled engineering of a one-pot spraying for Na‐ion batteries. The one‐pot spraying process directly adjusts the electrode structures process for Na-ion batteries. The one-pot spraying process directly adjusts the electrode from dense to porous by adjusting the spraying suspension concentration. The critical structures from dense to porous by adjusting the spraying suspension concentration. The suspension concentration (6.0 g/L) drives the direct layer‐by‐layer deposition of enlarged critical suspension concentration (6.0 g/L) drives the direct layer-by-layer deposition of aggregations on the substrate to make the electrode structure with the vacant areas rang‐ enlarged aggregations on the substrate to make the electrode structure with the vacant ing from bottom to top. As a result, the open sponge‐like electrode structure realizes areas ranging from bottom to top. As a result, the open sponge-like electrode structure higher specific capacity, rate capability, and capacity retention compared to the dense electrode (0.5 g/L), which can be induced by the improved diffusion coefficient and en‐ riched electroactive sites in the electrochemical reactions of the Na ions with the electrode interfaces. In addition, the high‐rate cycling capability of the open sponge‐like electrode is noted in both the specific (100.1 mAh/g and 90.2% cycling retention after 100 cycles at 5 C). The superior electrochemical performance is attributed to the efficient and stablePDF Image | Cathode Electrodes High-Rate Cycle-Stable Na-Ion Batteries
PDF Search Title:
Cathode Electrodes High-Rate Cycle-Stable Na-Ion BatteriesOriginal File Name Searched:
batteries-08-00181-v3.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |